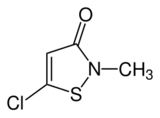
Chemistry:Methylchloroisothiazolinone
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Chloro-2-methyl-1,2-thiazol-3(2H)-one | |
| Other names
5-Chloro-2-methylisothiazol-3(2H)-one
5-Chloro-2-methyl-4-isothiazolin-3-one Chloromethylisothiazolinone Chloromethylisothiazolone Methylchloroisothiazolinone Methylchloroisothiazolone CMI CMIT MCI MCIT CIT | |
| Identifiers | |
3D model (JSmol)
|
|
| 1210149 | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H4ClNOS | |
| Molar mass | 149.59 g·mol−1 |
| Appearance | white solid |
| Density | 1.02 g/cm3 |
| Melting point | 52 °C (126 °F; 325 K) |
| Miscible | |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H300, H301, H310, H311, H314, H317, H330, H331, H335, H410 | |
| P260, P261, P262, P264, P270, P271, P272, P273, P280, P284, P301+310, P301+330+331, P302+350, P302+352, P303+361+353, P304+340, P305+351+338, P310, P311, P312, P320, P321, P322, P330, P333+313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylchloroisothiazolinone, also referred to as MCI, is the organic compound with the formula S(C2HCl)C(O)N(CH3). It is a white solid that melts near room temperature. The compound is an isothiazolinone, a class of heterocycles used as biocides. These compounds have an active sulphur moiety that is able to oxidize thiol-containing residues, thereby effectively killing most aerobic and anaerobic bacteria. MCI is often used in combination with methylisothiazolinone, a mixture known as Kathon. The isothiazolinones have attracted attention because they can cause contact dermatitis.[1][2][3] Methylchloroisothiazolinone is effective against gram-positive and gram-negative bacteria, yeast, and fungi.
Application
Methylchloroisothiazolinone is found in many water-based personal care products and cosmetics.[2] Methylchloroisothiazolinone was first used in cosmetics in the 1970s. It is also used in glue production, detergents, paints, fuels, and other industrial processes. Methylchloroisothiazolinone is known by the registered tradename Kathon CG when used in combination with methylisothiazolinone.[3]
Methylchloroisothiazolinone may be used in combination with other preservatives including ethylparaben, benzalkonium chloride, bronopol and phenoxyethanol.
Hazards
Methylchloroisothiazolinone can cause allergic reactions in some people.[4] The first publication of the preservative as a contact allergen was in 1988.[5] Cases of photoaggravated allergic contact dermatitis, i.e. worsening of skin lesions after sun exposure, have also been reported.[4]
In pure form or in high concentrations, methylchloroisothiazolinone is a skin and membrane irritant and causes chemical burns. In the United States, maximum authorized concentrations are 15 ppm in rinse-offs (of a mixture in the ratio 3:1 of 5-chloro-2-methylisothiazol 3(2H)-one and 2-methylisothiazol-3 (2H)-one).[6] In Canada, methylchloroisothiazolinone may only be used in rinse-off products in combination with methylisothiazolinone, the total concentration of the combination may not exceed 15 ppm.[7]
References
- ↑ Silva, Vânia; Silva, Cátia; Soares, Pedro; Garrido, E. Manuela; Borges, Fernanda; Garrido, Jorge (2020). "Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles". Molecules 25 (4): 991. doi:10.3390/molecules25040991. PMID 32102175.
- ↑ 2.0 2.1 Reinhard (2001). "Preservation of products with MCI/MI in Switzerland". Contact Dermatitis 45 (5): 257–264. doi:10.1034/j.1600-0536.2001.450501.x. PMID 11722483.
- ↑ 3.0 3.1 Knudsen BB, Menne T (1990). "Kathon CG--a new contact sensitizing preservative". Ugeskrift for Lægerer 152 (10): 656–657. PMID 2321281.
- ↑ 4.0 4.1 Pirmez, R.; Fernandes, A.L.C.; Melo, M.G.M. (2015). "Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/Methylisothiazolinone): A novel pattern of involvement in a growing epidemic?". British Journal of Dermatology 173 (5): 1343–1344. doi:10.1111/bjd.13986. PMID 26130214.
- ↑ De Groot, A. C.; Weyland, J. W. (1988). "Kathon CG: A review". Journal of the American Academy of Dermatology 18 (2 Pt 1): 350–358. doi:10.1016/s0190-9622(88)70051-1. PMID 3279090.
- ↑ "Annex VI release - 26 November 2017 - 201703". https://ww2.fda.gov.ph/attachments/article/15926/Annex%20VI%20release%20-%2026%20November%202017%20-%20201703.pdf.
- ↑ "Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients". 2004-06-18. http://www.hc-sc.gc.ca/cps-spc/cosmet-person/hot-list-critique/hotlist-liste-eng.php.
External links
- CMIT/MIT Assessment
- Methylchloroisothiazoline in the Consumer Product Information Database
- Methylchloroisothiazolinone at the National Library of Medicine
 |

