Chemistry:Methylhippuric acid
From HandWiki
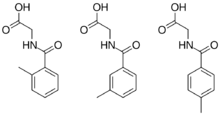 Chemical structures of 2-, 3-, and 4-methylhippuric acid
| |
| Names | |
|---|---|
| Other names
Toluric acid; N-(Toluoyl)glycine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C10H11NO3 | |
| Molar mass | 193.202 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylhippuric acid is a carboxylic acid and organic compound. Methylhippuric acid has three isomers. The isomers include 2-, 3-, and 4-methylhippuric acid.[1]
Methylhippuric acids are metabolites of the isomers of xylene.[1][2] The presence of methylhippuric acid can be used as a biomarker to determine exposure to xylene.[2][3]
See also
References
- ↑ 1.0 1.1 "HIPPURIC and METHYL HIPPURIC ACIDS in urine". NIOSH Manual of Analytical Methods (NMAM). https://www.cdc.gov/niosh/docs/2003-154/pdfs/8301.pdf.
- ↑ 2.0 2.1 Inoue, O.; Seiji, K.; Kawai, T.; Watanabe, T.; Jin, C.; Cai, S. X.; Chen, Z.; Qu, Q. S. et al. (1993). "Excretion of methylhippuric acids in urine of workers exposed to a xylene mixture: Comparison among three xylene isomers and toluene". International Archives of Occupational and Environmental Health 64 (7): 533–9. doi:10.1007/bf00381104. PMID 8482596.
- ↑ Kira S (1977). "Measurement by gas chromatography of urinary hippuric acid and methylhippuric acid as indices of toluene and xylene exposure". Occupational and Environmental Medicine 34 (305–309): 305–309. doi:10.1136/oem.34.4.305. PMID 588486.
 |

