Chemistry:Hippuric acid
| |

| |
| Names | |
|---|---|
| IUPAC name
N-Benzoylglycine
| |
| Preferred IUPAC name
Benzamidoacetic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H9NO3 | |
| Molar mass | 179.175 g·mol−1 |
| Density | 1.371 g/cm3 |
| Melting point | 187 to 188 °C (369 to 370 °F; 460 to 461 K) |
| Boiling point | 240 °C (464 °F; 513 K) (decomposes) |
| Hazards | |
| Safety data sheet | Material Safety Data Sheet |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hippuric acid (Gr. hippos, horse, ouron, urine) is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenolic compounds (such as in fruit juice, tea and wine). The phenols are first converted to benzoic acid, and then to hippuric acid and excreted in urine.[1]
Hippuric acid crystallizes in rhombic prisms which are readily soluble in hot water, melt at 187 °C, and decompose at about 240 °C.[2] High concentrations of hippuric acid may also indicate a toluene intoxication; however, scientists have called this correlation into question because there are other variables that affect levels of hippuric acid.[3] When many aromatic compounds such as benzoic acid and toluene are taken internally, they are converted to hippuric acid by reaction with the amino acid, glycine.
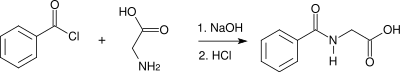
Synthesis
A modern synthesis of hippuric acid involves the acylation of glycine with benzoyl chloride ("Schotten–Baumann reaction").[4]
Physiology
Biochemically, hippuric acid is produced from benzoic acid and glycine, which occurs in the liver, intestine, and kidneys.[5] In terms of mechanism, benzoic acid is converted to benzoyl CoA, an acylating agent.[6]
Hippuric acid may be formed from the essential amino acid phenylalanine through at least two pathways. Phenylalanine undergoes biotransformation to form an alpha-keto acid, phenylpyruvic acid, which can tautomerize to a reactive enol. The benzylic carbon is reactive which undergoes peroxidation followed by the competing pathways to either react with the alpha carbon subsequently form an dioxetanol intermediate followed by formation of oxalic acid and benzaldehyde, or, peroxidation can react with the carboxyl group to form an alpha-keto-beta-peroxylactone intermediate followed by formation of carbon monoxide, carbon dioxide, and benzaldehyde. Alternatively, under certain conditions, phenylpyruvic acid may undergo a redox mechanism, such as Iron(II) donating an electron, to directly release carbon dioxide, followed by carbon monoxide, for the formation of a stable toluene radical which is resolved by an antioxidant such as ascorbate. In all of the aforementioned cases, benzaldehyde undergoes biotransformation via CYP450 to benzoic acid followed by conjugation to glycine for formation of hippurate which undergoes urinary excretion.[7] Similarly, toluene reacts with CYP450 to form benzaldehyde.[8]
Hippuric acid has been reported to be a marker for Parkinson's disease.[9]
Reactions
Hippuric acid is readily hydrolysed by hot caustic alkalis to benzoic acid and glycine. Nitrous acid converts it into benzoyl glycolic acid, C6H5C(=O)OCH2CO2H. Its ethyl ester reacts with hydrazine to form hippuryl hydrazine, C6H5CONHCH2CONHNH2, which was used by Theodor Curtius for the preparation of hydrazoic acid.[2]
History
Justus von Liebig showed in 1829 that hippuric acid differed from benzoic acid and he named it,[10] and in 1834 he determined its constitution,[11] while in 1853 French chemist Victor Dessaignes (1800–1885) synthesized it by the action of benzoyl chloride on the zinc salt of glycine.[12] It was also formed by heating benzoic anhydride with glycine,[13] and by heating benzamide with monochloroacetic acid.[2]
See also
- para-Aminohippuric acid
- ortho-Iodohippuric acid
- Methylhippuric acid (three different isomers)
References
- ↑ Wishart, David S.; Djombou Feunang, Yannick; Marcu, Ana; Guo, An Chi; Liang, Kevin; Vázquez Fresno, Rosa; Sajed, Tanvir; Johnson, Daniel et al.. "Showing metabocard for Hippuric acid (HMDB0000714)". http://www.hmdb.ca/metabolites/HMDB0000714.
- ↑ 2.0 2.1 2.2
 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Hippuric Acid". Encyclopædia Britannica. 13 (11th ed.). Cambridge University Press. p. 523.
One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Hippuric Acid". Encyclopædia Britannica. 13 (11th ed.). Cambridge University Press. p. 523.
- ↑ Pero, RW (2010). "Health consequences of catabolic synthesis of hippuric acid in humans". Current Clinical Pharmacology 5 (1): 67–73. doi:10.2174/157488410790410588. PMID 19891605.
- ↑ Ingersoll, A. W.; Babcock, S. H. (1932). "Hippuric acid". Organic Syntheses 12: 40. doi:10.15227/orgsyn.012.0040. http://www.orgsyn.org/demo.aspx?prep=cv2p0328.; Collective Volume, 2, pp. 328
- ↑ "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. March 2009. doi:10.1073/pnas.0812874106. PMID 19234110. Bibcode: 2009PNAS..106.3698W.
- ↑ Chiba, M.; Poon, K.; Hollands, J.; Pang, K. S. (1994). "Glycine Conjugation Activity of Benzoic Acid and its Acinar Localization in the Perfused Rat Liver". The Journal of Pharmacology and Experimental Therapeutics 268 (1): 409–416. PMID 8301581.
- ↑ Hopper, Christopher P.; De La Cruz, Ladie Kimberly; Lyles, Kristin V.; Wareham, Lauren K.; Gilbert, Jack A.; Eichenbaum, Zehava; Magierowski, Marcin; Poole, Robert K. et al. (2020-12-23). "Role of Carbon Monoxide in Host–Gut Microbiome Communication" (in en). Chemical Reviews 120 (24): 13273–13311. doi:10.1021/acs.chemrev.0c00586. ISSN 0009-2665. PMID 33089988.
- ↑ Hopper, Christopher P.; Zambrana, Paige N.; Goebel, Ulrich; Wollborn, Jakob (2021). "A brief history of carbon monoxide and its therapeutic origins" (in en). Nitric Oxide 111-112: 45–63. doi:10.1016/j.niox.2021.04.001. ISSN 1089-8603. PMID 33838343.
- ↑ "Parkinson's smell test explained by science". BBC News (BBC). 20 March 2019. https://www.bbc.com/news/uk-scotland-47627179.
- ↑ Liebig, Justus (1829). "Ueber die Säure, welche in dem Harn der grasfressenden vierfüssigen Thiere enthalten ist" (in German). Annalen der Physik und Chemie 17 (11): 389–399. doi:10.1002/andp.18290931104. Bibcode: 1829AnP....93..389L. https://zenodo.org/record/1883346. Liebig named hippuric acid on p. 390: "Da ich die Säure aus dem Pferdeharn vorzugsweise untersucht habe, so werde ich sie, in Ermanglung eines passenderen Namens, mit Hippursäure bezeichnen." (Since I have especially investigated the acid from horse urine, then, for want of a more suitable name, I will designate it with [the name] "hippuric acid".)
- ↑ Liebig, Justus (1834) "Ueber die Zusammensetzung der Hippursäure" (On the composition of hippuric acid), Annalen der Physik und Chemie, 32 : 573–574.
- ↑ Dessaignes V. (1853). "Ueber die Regeneration der Hippursäure". Annalen der Chemie und Pharmacie 87 (3): 325–327. doi:10.1002/jlac.18530870311. https://babel.hathitrust.org/cgi/pt?id=uva.x002457940;view=1up;seq=337. See also: Dessaignes (1853) "Note sur la régénération de l'acide hipparique," Comptes rendus, 37 : 251–252.
- ↑ Curtius T. (1884). "Synthese von Hippursäure und Hippursäureäthern". Berichte der Deutschen Chemischen Gesellschaft 17 (2): 1662–1663. https://zenodo.org/record/1425365.
 |