Chemistry:N-Acetyllactosamine
From HandWiki
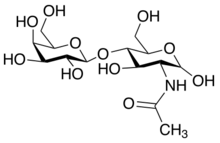
| |
| Names | |
|---|---|
| IUPAC name
N-[β-D-Galactopyranosyl-(1→4)-(2-deoxy-D-glucos-2-yl)]acetamide
| |
| Systematic IUPAC name
N-[(2R,3R,4S,5R)-3,5,6-Trihydroxy-1-oxo-4-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}hexan-2-yl]acetamide | |
| Other names
β-D-galactopyranosyl-(1→4)-2-acetamido-2-deoxy-β-D-glucopyranose; 2-(Acetylamino)-2-deoxy-4-O-hexopyranosylhexopyranose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H25NO11 | |
| Molar mass | 383.350 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Acetyllactosamine (LacNAc) (also known as CD75) is a nitrogen-containing disaccharide,[1] a lactosamine derivative that is substituted with an acetyl group on its glucosamine component.
The N-acetyllactosamine is a component of many glycoproteins[2] and functions as a carbohydrate antigen that is thought to play roles in normal cellular recognition as well as in malignant transformation and metastasis.[3] It is also found in the structure of human milk oligosaccharides and has prebiotic effects.[4]
References
- ↑ "Isolation of N-Acetyllactosamine and Galactosyl-β-D-(1 → 4)-N-acetyllactosamine from Beef Brain Glycopeptides". The Journal of Biological Chemistry 247 (12): 3744–9. June 1972. doi:10.1016/S0021-9258(19)45097-7. PMID 5033387. http://www.jbc.org/content/247/12/3744.full.pdf.
- ↑ "Why are glycoproteins modified by poly-N-acetyllactosamine glyco-conjugates?". Current Protein & Peptide Science 4 (1): 1–9. February 2003. doi:10.2174/1389203033380304. PMID 12570780.
- ↑ "Histochemical demonstration and analysis of poly-N-acetyllactosamine structures in normal and malignant human tissues". Histology and Histopathology 11 (1): 203–14. January 1996. PMID 8720464.
- ↑ "Simulation and economic assessment of large-scale enzymatic N-acetyllactosamine manufacture". Biochemical Engineering Journal 154: 107459. February 2020. doi:10.1016/j.bej.2019.107459. https://backend.orbit.dtu.dk/ws/files/203285336/bsog_1_s2.0_S1369703X19303985_main.pdf.
External links
- N-acetyllactosamine at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

