Chemistry:Acetyl group
| |
| Names | |
|---|---|
| IUPAC name | |
| Systematic IUPAC name
Methyloxidocarbon(•)[4] (additive) | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | Ac |
| 1697938 | |
| ChEBI | |
| ChemSpider | |
| 786 | |
PubChem CID
|
|
| |
| |
| Properties | |
| C2H3O | |
| Molar mass | 43.045 g·mol−1 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−15 to −9 kJ mol−1 |
| Related compounds | |
Related compounds
|
Acetone Carbon monoxide Acetic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
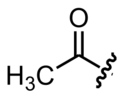
In organic chemistry, an acetyl group is a functional group denoted by the chemical formula –COCH
3 and the structure –C(=O)–CH
3. It is sometimes represented by the symbol Ac[5][6] (not to be confused with the element actinium). In IUPAC nomenclature, an acetyl group is called an ethanoyl group.
An acetyl group contains a methyl group (–CH
3) that is single-bonded to a carbonyl (C=O), making it an acyl group. The carbonyl center of an acyl radical has one non-bonded electron with which it forms a chemical bond to the remainder (denoted with the letter R) of the molecule.
The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin).
Acetylation
Acetylation is the chemical reaction known as "ethanoylation" in the IUPAC nomenclature. It depicts a reactionary process that injects an acetyl functional group into a chemical compound. The opposite reaction is called "deacetylation", and this is the removal of the acetyl group. An example of an acetylation reaction is the conversion of glycine to N-acetylglycine:[7]
- H
2NCH
2CO
2H + (CH
3CO)
2O → CH
3C(O)NHCH
2CO
2H + CH
3CO
2H
In biology
Enzymes which perform acetylation on proteins or other biomolecules are known as acetyltransferases. In biological organisms, acetyl groups are commonly transferred from acetyl-CoA to other organic molecules. Acetyl-CoA is an intermediate in the biological synthesis and in the breakdown of many organic molecules. Acetyl-CoA is also created during the second stage of cellular respiration (pyruvate decarboxylation) by the action of pyruvate dehydrogenase on pyruvic acid.[8]
Proteins are often modified via acetylation, for various purposes. For example, acetylation of histones by histone acetyltransferases (HATs) results in an expansion of local chromatin structure, allowing transcription to occur by enabling RNA polymerase to access DNA. However, removal of the acetyl group by histone deacetylases (HDACs) condenses the local chromatin structure, thereby preventing transcription.[9]
In synthetic organic and pharmaceutical chemistry
Acetylation can be achieved by chemists using a variety of methods, most commonly with the use of acetic anhydride or acetyl chloride, often in the presence of a tertiary or aromatic amine base.
Pharmacology
Acetylated organic molecules exhibit increased ability to cross the selectively permeable blood–brain barrier.[10] Acetylation helps a given drug reach the brain more quickly, making the drug's effects more intense and increasing the effectiveness of a given dose. The acetyl group in acetylsalicylic acid (aspirin) enhances its effectiveness relative to the natural anti-inflammatant salicylic acid. In similar manner, acetylation converts the natural painkiller morphine into the far more potent heroin (diacetylmorphine).[10]
There is some evidence that acetyl-L-carnitine may be more effective for some applications than L-carnitine.[11] Acetylation of resveratrol holds promise as one of the first anti-radiation medicines for human populations.[12]
Etymology
The term "acetyl" was coined by the German chemist Justus von Liebig in 1839 to describe what he incorrectly believed to be the radical of acetic acid (the main component of vinegar, aside from water), which is now known as the vinyl group (coined in 1851); "acetyl" is derived from the Latin acētum, meaning "vinegar." When it was shown that Liebig's theory was wrong and acetic acid had a different radical, his name was carried over to the correct one, but the name of acetylene (coined in 1860) was retained.[13]
See also
- Acetaldehyde
- Acetoxy group
- Histone acetylation and deacetylation
- Polyoxymethylene plastic (acetal resin), a thermoplastic
References
- ↑ "List of Radical Names Beginning from "A"". Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford, 1979. Copyright 1979 IUPAC. http://www.acdlabs.com/iupac/nomenclature/79/r79_1037.htm.
- ↑ "R-5.7.1 Carboxylic acids, where acetyl appears as an example". IUPAC, Commission on Nomenclature of Organic Chemistry. A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993), 1993, Blackwell Scientific publications, Copyright 1993 IUPAC. http://www.acdlabs.com/iupac/nomenclature/93/r93_480.htm.
- ↑ IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-65.1.7.2.1". in Favre, Henri A.; Powell, Warren H.. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4. https://pubs.rsc.org/en/Content/eBook/978-0-85404-182-4.
- ↑ "Acetyl". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=46887.
- ↑ Banik, Gregory M., ed (January 2020) (in en). The ACS Guide to Scholarly Communication. Washington, DC: American Chemical Society. doi:10.1021/acsguide.50308. ISBN 978-0-8412-3586-1. https://pubs.acs.org/doi/book/10.1021/acsguide.
- ↑ Hanson, James A. (2001). Functional group chemistry. Cambridge, Eng: Royal Society of Chemistry. pp. 11. ISBN 0-85404-627-5.
- ↑ Herbst, R. M.; Shemin, D. (1943). "Acetylglycine". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV2P0011.; Collective Volume, 2, pp. 11
- ↑ Patel, Mulchand (June 13, 2014). "The Pyruvate Dehydrogenase Complexes: Structure-based Function and Regulation". The Journal of Biological Chemistry 289 (24): 16615–16623. doi:10.1074/jbc.R114.563148. PMID 24798336.
- ↑ Nelson, David L.; Cox, Michael M. (2000). Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. ISBN 1-57259-153-6. https://archive.org/details/lehningerprincip01lehn.
- ↑ 10.0 10.1 Pardridge, William M (2012-08-29). "Drug Transport across the Blood–Brain Barrier" (in en). Journal of Cerebral Blood Flow & Metabolism 32 (11): 1959–1972. doi:10.1038/jcbfm.2012.126. ISSN 0271-678X. PMID 22929442.
- ↑ Liu, J; Head, E; Kuratsune, H; Cotman, C. W.; Ames, B. N. (2004). "Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats". Annals of the New York Academy of Sciences 1033 (1): 117–31. doi:10.1196/annals.1320.011. PMID 15591009. Bibcode: 2004NYASA1033..117L.
- ↑ Koide, Kazunori; Osman, Sami; Garner, Amanda L.; Song, Fengling; Dixon, Tracy; Greenberger, Joel S.; Epperly, Michael W. (14 April 2011). "The Use of 3,5,4′-Tri-acetylresveratrol as a Potential Prodrug for Resveratrol Protects Mice from γ-Irradiation-Induced Death". ACS Medicinal Chemistry Letters 2 (4): 270–274. doi:10.1021/ml100159p. PMID 21826253.
- ↑ Constable, Edwin C.; Housecroft, Catherine E. (2020-04-20). "Before Radicals Were Free – the Radical Particulier of de Morveau" (in en). Chemistry 2 (2): 293–304. doi:10.3390/chemistry2020019. ISSN 2624-8549.
 |
