Chemistry:N-Acetyltaurine
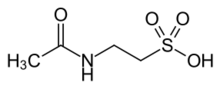
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Acetamidoethane-1-sulfonic acid | |
| Other names
NAcT
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H9NO4S | |
| Molar mass | 167.18 g·mol−1 |
| Appearance | Solid |
| Soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Acetyltaurine (NAcT) is an endogenous metabolite. Biochemically, N-acetyltaurine is formed as a result of an acetylation of taurine. The main substrate for this reaction is acetate.[1] An increase of endogenous N-acetyltaurine concentrations was observed after the consumption of alcohol and after extended physical activity (ketoacidosis).[2][3][4]
History
N-Acetyltaurine was first mentioned in 1990 as a compound in the droplets of the orb spider's viscid spiral.[5] Based on its high hygroscopicity, N-acetyltaurine is an important ingredient which ensures the spider web's flexibility.
As a biomarker for ethanol metabolism, N-acetyltaurine was first mentioned in a mice study in 2012.[1] Another study in 2015 focused on the effect of endurance training on an increase in N-acetyltaurine concentrations.[2] The first study focusing on the forensic context of alcohol biomarker analysis in human urine was published in 2016.[4] One year later, in 2017 an evaluation of N-acetyltaurine as an alcoholmarker in human blood followed.[3]
Significance as an alcohol marker
N-Acetyltaurine is a direct alcohol biomarker which represents the oxidative pathway of ethanol metabolism. Other direct alcohol biomarkers such as fatty acid ethyl esters (FAEE), ethyl glucuronide, ethyl sulfate, and phosphatidylethanol reflect the non-oxidative pathway of alcohol metabolism, based on conjunction reactions (biotransformation).
The fact that N-acetyltaurine is an endogenous metabolite reduces its significance as an alcohol biomarker: A distinction between endogenous N-acetyltaurine concentrations and alcohol induced concentrations is necessary.
During a drinking study with a target blood alcohol concentrations of 0.8 g/kg, an alcohol induced concentration of N-acetyltaurine which was about ten folds higher than the endogenous concentration was observed.[4] In blood the alcohol induced increase was only twofold. [3] Based on these observations, it was concluded that N-acetyltaurine is excreted very efficiently by the kidney.
Analytics
N-Acetyltaurine can be quantified by a combination of high performance liquid chromatography and mass spectrometry (MS/MS). Due to the high hydrophilicity of N-acetyltaurine, the hydrophilic interaction chromatography (HILIC) is the method of choice in order to separate the analyte from the matrix components.[3][4]
References
- ↑ 1.0 1.1 "Identification of N-acetyltaurine as a novel metabolite of ethanol through metabolomics-guided biochemical analysis". The Journal of Biological Chemistry 287 (9): 6336–49. February 2012. doi:10.1074/jbc.m111.312199. PMID 22228769.
- ↑ 2.0 2.1 "Increased N-Acetyltaurine in the Skeletal Muscle After Endurance Exercise in Rat". Taurine 10. Advances in Experimental Medicine and Biology. 975. 2017. pp. 403–411. doi:10.1007/978-94-024-1079-2_33. ISBN 978-94-024-1077-8.
- ↑ 3.0 3.1 3.2 3.3 "Evaluation of N-acetyltaurine as an ethanol marker in human blood". Alcohol 65: 11–18. December 2017. doi:10.1016/j.alcohol.2017.05.007. PMID 29084624.
- ↑ 4.0 4.1 4.2 4.3 "N-Acetyltaurine as a novel urinary ethanol marker in a drinking study". Analytical and Bioanalytical Chemistry 408 (26): 7529–36. October 2016. doi:10.1007/s00216-016-9855-7. PMID 27520321. https://boris.unibe.ch/86011/1/art%253A10.1007%252Fs00216-016-9855-7.pdf.
- ↑ Vollrath, Fritz; Fairbrother, Wayne J; Williams, Robert J. P; Tillinghast, Edward K; Bernstein, David T; Gallagher, Kathleen S; Townley, Mark A (1990). "Compounds in the droplets of the orb spider's viscid spiral". Nature 345 (6275): 526–8. doi:10.1038/345526a0. Bibcode: 1990Natur.345..526V.
External links
"N-Acetyltaurine as a novel urinary ethanol marker in a drinking study". Analytical and Bioanalytical Chemistry 408 (26): 7529–36. October 2016. doi:10.1007/s00216-016-9855-7. PMID 27520321. https://boris.unibe.ch/86011/1/art%253A10.1007%252Fs00216-016-9855-7.pdf.
 |

