Chemistry:Phosphatidylethanol
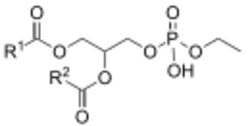
Phosphatidylethanols (PEth) are a group of phospholipids formed only in the presence of ethanol via the action of phospholipase D (PLD).[1] It accumulates in blood and is removed slowly, making it a useful biomarker for alcohol consumption.[2] PEth is also thought to contribute to the symptoms of alcohol intoxication.[3]
Structure
Chemically, phosphatidylethanols are phospholipids carrying two fatty acid chains, which are variable in structure, and one phosphate ethyl ester.
Biosynthesis
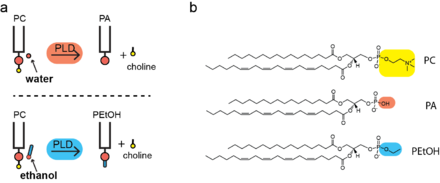
When ethanol is present, PLD substitutes ethanol for water and covalently attaching the alcohol as the head group of the phospholipid; hence the name phosphatidylethanol. Normally PLD incorporates water to generate phosphatidic acid (PA); the process is termed transphosphatidylation.[4] PLD continues to generate PA in the presence of ethanol and while PEth is generated and the effects of ethanol transphosphatidlyation are through the generation of the unnatural lipid not depletion of PA.[3]
Biological effects
The lipid accumulates in the human body and competes at agonists sites of lipid-gated ion channels contributing to alcohol intoxication.[3] The chemical similarity of PEth to phosphatidic acid (PA) and phosphatidylinositol 4,5-bisphosphate (PIP2) suggest a likely broad perturbation to lipid signaling; the exact role of PEth as a competitive lipid ligand has not been studied extensively.
Marker in blood
Levels of phosphatidylethanols in blood are used as markers of previous alcohol consumption.[5][6] An increase of alcohol intake by ~20 g ethanol/day will raise the PEth 16:0/18:1 concentration by ~0.10 μmol/L, and vice versa if the alcohol consumption has decreased. However, it has been demonstrated that there can be significant inter-personal variation, leading to potential misclassification between moderate and heavy drinkers.[7] After cessation of alcohol intake, the half-life of PEth is between 4.5 and 10 days in the first week and between 5 and 12 days in the second week.[2] As a blood marker PEth is more sensitive than carbohydrate deficient transferrin (CDT), urinary ethyl glucuronide (EtG) and ethyl sulfate (EtS).[8]
Interpretation
The Society of PEth Research[9] published a harmonization document (2022 Consensus of Basel) for the interpretation of phosphatidylethanol concentrations in the clinical and forensic setting.[10] This consensus represents the first internationally established harmonization document on PEth and was created by an assembly of the world's leading experts in phosphatidylethanol research. The consensus defines the target measurand (PEth 16:0/18:1 in whole blood), cutoff concentrations (20 ng/mL and 200 ng/mL), and minimal requirements for the applied analytical method (accuracy and precision within 15%).
References
- ↑ Gnann, H.; Engelmann, C.; Skopp, G.; Winkler, M.; Auwärter, V.; Dresen, S.; Ferreirós, N.; Wurst, F. M. et al. (2010). "Identification of 48 homologues of phosphatidylethanol in blood by LC-ESI-MS/MS". Analytical and Bioanalytical Chemistry 396 (7): 2415–23. doi:10.1007/s00216-010-3458-5. PMID 20127079. https://boris.unibe.ch/1753/.
- ↑ 2.0 2.1 Kechagias, Stergios; Dernroth, Dženeta Nezirević; Blomgren, Anders; Hansson, Therese; Isaksson, Anders; Walther, Lisa; Kronstrand, Robert; Kågedal, Bertil et al. (2015). "Phosphatidylethanol Compared with Other Blood Tests as a Biomarker of Moderate Alcohol Consumption in Healthy Volunteers: A Prospective Randomized Study". Alcohol and Alcoholism 50 (4): 399–406. doi:10.1093/alcalc/agv038. ISSN 0735-0414. PMID 25882743.
- ↑ 3.0 3.1 3.2 Chung, HW; Petersen, EN; Cabanos, C; Murphy, KR; Pavel, MA; Hansen, AS; Ja, WW; Hansen, SB (18 January 2019). "A Molecular Target for an Alcohol Chain-Length Cutoff.". Journal of Molecular Biology 431 (2): 196–209. doi:10.1016/j.jmb.2018.11.028. PMID 30529033.
- ↑ Yang, SF; Freer, S; Benson, AA (10 February 1967). "Transphosphatidylation by phospholipase D.". The Journal of Biological Chemistry 242 (3): 477–84. doi:10.1016/S0021-9258(18)96298-8. PMID 6022844.
- ↑ Hansson, Per; Caron, Murielle; Johnson, Goran; Gustavsson, Lena; Alling, Christer (1997). "Blood Phosphatidylethanol as a Marker of Alcohol Abuse: Levels in Alcoholic Males during Withdrawal". Alcoholism: Clinical and Experimental Research 21 (1): 108–110. doi:10.1111/j.1530-0277.1997.tb03736.x. PMID 9046381.
- ↑ Hansson, P; Varga, A; Krantz, P; Alling, C (2001). "Phosphatidylethanol in post-mortem blood as a marker of previous heavy drinking". International Journal of Legal Medicine 115 (3): 158–61. doi:10.1007/s004140100206. PMID 11775018.
- ↑ Helander, Anders; Hermansson, Ulric; Beck, Olof (2019). "Dose–Response Characteristics of the Alcohol Biomarker Phosphatidylethanol (PEth)—A Study of Outpatients in Treatment for Reduced Drinking" (in en). Alcohol and Alcoholism 54 (6): 567–573. doi:10.1093/alcalc/agz064. ISSN 0735-0414. PMID 31529064. https://academic.oup.com/alcalc/article/54/6/567/5569509.
- ↑ Helander, A.; Peter, O.; Zheng, Y. (2012). "Monitoring of the Alcohol Biomarkers PEth, CDT and EtG/EtS in an Outpatient Treatment Setting". Alcohol and Alcoholism 47 (5): 552–557. doi:10.1093/alcalc/ags065. ISSN 0735-0414. PMID 22691387.
- ↑ "The PEth-NET News". https://peth-net.org/. Retrieved 1 April 2023.
- ↑ Luginbühl, Marc; Wurst, Friedrich M.; Stöth, Frederike; Weinmann, Wolfgang; Stove, Christophe P.; Van Uytfanghe, Katleen (18 July 2022). "Consensus for the use of the alcohol biomarker phosphatidylethanol (PEth) for the assessment of abstinence and alcohol consumption in clinical and forensic practice (2022 Consensus of Basel)". Drug Testing and Analysis 14 (10): 1800–1802. doi:10.1002/dta.3340. PMID 35851997. https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/dta.3340.
 |

