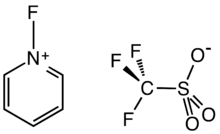
Chemistry:N-Fluoropyridinium triflate
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Fluoropyridin-1-ium trifluoromethanesulfonate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H5F4NO3S | |
| Molar mass | 247.16 g·mol−1 |
| Appearance | White solid |
| Melting point | 185–187 °C (365–369 °F; 458–460 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Fluoropyridinium triflate is an organofluorine compound with the formula [C5H5NF]O3SCF3. It is a white solid with low solubility in polar organic solvents. The compound is used as an electrophilic fluorinating agent. It is a salt, consisting of the N-fluoropyridinium cation ([C5H5NF]+) and the triflate anion.[1] Related reagents include Selectfluor, which is also an N-fluorinated salt.
N-Fluoropyridinium cations are not only electrophilic fluorinating agents (i.e., sources of "F+"), they are also one-electron oxidants.[2]
References
- ↑ Teruo Umemoto, Ahmad El-Awa "N-Fluoropyridinium Triflate" e-EROS Encyclopedia of Reagents for Organic Synthesis. Published Online: 22 APR 2013 doi:10.1002/047084289X.rf012.pub2
- ↑ Kiselyov, A. S., "Chemistry of N-fluoropyridinium salts", Chemical Society Reviews 2005, vol. 34, page 1031. doi:10.1039/B509217P
 |

