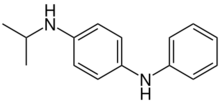
Chemistry:N-Isopropyl-N'-phenyl-1,4-phenylenediamine
| |
| Names | |
|---|---|
| Preferred IUPAC name
N1-Phenyl-N4-(propan-2-yl)benzene-1,4-diamine | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1673 |
| |
| |
| Properties | |
| C15H18N2 | |
| Molar mass | 226.323 g·mol−1 |
| Appearance | dark grey flakes |
| Density | 1.04 |
| Melting point | 75 °C (167 °F; 348 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H302, H317, H320, H371, H372, H373, H410 | |
| P260, P261, P264, P270, P272, P273, P280, P301+312, P302+352, P305+351+338, P309+311, P314, P321, P330, P333+313, P337+313, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Isopropyl-N′-phenyl-1,4-phenylenediamine (often abbreviated IPPD) is an organic compound commonly used as an antiozonant in rubbers.[1] Like other p-phenylenediamine-based antiozonants it works by virtue of its low ionization energy, which allows it to react with ozone faster than ozone will react with rubber.[2] This reaction converts it to the corresponding aminoxyl radical (R2N–O•), with the ozone being converted to a hydroperoxyl radical (HOO•),[2] these species can then be scavenged by other antioxidant polymer stabilizers.
IPPD is prone to process called blooming, where it migrates to the surface of the rubber.[3] This can be beneficial to the tire, as ozone attacks the tire surface and blooming therefore moves the antiozonant to where it is most needed,[4] however this also increases the leaching of IPPD into the environment. Many tire producers have moved to using 6PPD instead, as this migrates more slowly. Oxidation of IPPD converts the central phenylenediamine ring into a quinone.[5]
Safety
IPPD is a human allergen.[6][7] It is the compound responsible for coining the term "Volkswagen Dermatitis".[8] There is some preliminary evidence for it being harmful to fish.[9]
See also
- N,N′-Di-2-butyl-1,4-phenylenediamine - a phenylenediamine based antioxidant used as a fuel additive
References
- ↑ Lewis, P.M. (January 1986). "Effect of ozone on rubbers: Countermeasures and unsolved problems". Polymer Degradation and Stability 15 (1): 33–66. doi:10.1016/0141-3910(86)90004-2.
- ↑ 2.0 2.1 Cataldo, Franco (January 2018). "Early stages of p-phenylenediamine antiozonants reaction with ozone: Radical cation and nitroxyl radical formation". Polymer Degradation and Stability 147: 132–141. doi:10.1016/j.polymdegradstab.2017.11.020.
- ↑ Choi, Sung-Seen (5 July 1997). "Migration of Antidegradants to the Surface in NR and SBR Vulcanizates". Journal of Applied Polymer Science 65 (1): 117–125. doi:10.1002/(SICI)1097-4628(19970705)65:1<117::AID-APP15>3.0.CO;2-0.
- ↑ Ignatz-Hoover, Frederick; To, Byron H.; Datta, R. N.; De Hoog, Arie J.; Huntink, N. M.; Talma, A. G. (1 July 2003). "Chemical Additives Migration in Rubber". Rubber Chemistry and Technology 76 (3): 747–768. doi:10.5254/1.3547765.
- ↑ Cao, Guodong; Wang, Wei; Zhang, Jing; Wu, Pengfei; Zhao, Xingchen; Yang, Zhu; Hu, Di; Cai, Zongwei (5 April 2022). "New Evidence of Rubber-Derived Quinones in Water, Air, and Soil". Environmental Science & Technology 56 (7): 4142–4150. doi:10.1021/acs.est.1c07376. PMID 35316033. Bibcode: 2022EnST...56.4142C.
- ↑ Lammintausta, K; Kalimo, K (1985). "Sensitivity to Rubber. Study with Rubber Mixes and Individual Rubber Chemicals.". Dermatosen in Beruf und Umwelt. Occupation and Environment 33 (6): 204–8. PMID 2936592.
- ↑ Conde-Salazar, Luis; del-Río, Emilio; Guimaraens, Dolores; Domingo, Antonia González (August 1993). "Type IV Allergy to Rubber Additives: A 10-Year Study of 686 Cases". Journal of the American Academy of Dermatology 29 (2): 176–180. doi:10.1016/0190-9622(93)70163-N. PMID 8335734.
- ↑ Jordan, William P. Jr. (1971-01-01). "Contact Dermatitis From N-Isopropyl-N-Phenylparaphenylenediamine: "Volkswagen Dermatitis"". Archives of Dermatology 103 (1): 85–87. doi:10.1001/archderm.1971.04000130087014. ISSN 0003-987X.
- ↑ Zhong, Liqiao; Peng, Weijuan; Liu, Chunsheng; Gao, Lei; Chen, Daqing; Duan, Xinbin (July 2022). "IPPD-induced growth inhibition and its mechanism in zebrafish". Ecotoxicology and Environmental Safety 239: 113614. doi:10.1016/j.ecoenv.2022.113614. PMID 35567929.
 |

