Chemistry:Naphthol Green B
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
trisodium; iron(3+); 5-nitroso-6-oxidonaphthalene-2-sulfonate
| |
| Other names
Acid Green 1; C.I. 10020
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H15FeN3Na3O15S3 | |
| Molar mass | 878.45 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
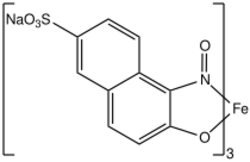
Naphthol Green B is a coordination complex of iron that is used as a dye.[1] The ligand is a sulfonated derivative of 1-nitroso-2-naphthol.
Structure
Naphthol Green B is the sodium salt of Naphthol Green Y (C.I. 10005). The organic ligands each bind to iron as bidentate ligands through the nitrogen and the anionic phenoxide groups. Three ligands are bound to the iron.[2]
Applications
Its absorption maximum is 714 nm in water.[3] It is water-soluble.
Naphthol Green B is used in histology to stain collagen.[4] Moreover, it is used for polychrome stains with animal tissue. For industry purposes Naphthol Green B is used for staining wool, nylon, paper, anoxidized aluminium and soap.[3]
References
- ↑ Raue, Roderich; Corbett, John F. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_383.
- ↑ Wang, Xiao; Zhang, Tianyong; Li, Bin; Yang, Qiusheng; Jiang, Shuang (2014). "Efficient Hydroxylation of Aromatic Compounds Catalyzed by an Iron(II) Complex with H2 O2". Applied Organometallic Chemistry 28 (9): 666–672. doi:10.1002/aoc.3178.
- ↑ 3.0 3.1 Horobin, RW. und Kiernan, JA. (2002): Conn's Biological Stains: A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine. BIOS Scientific Publ., 10th edition; ISBN:1-85996-099-5; page 101 and 102
- ↑ Histological and Histochemical Methods: Theory and Practice, 4th edition, J. A. Kiernan
External links
- Naphthol Green B at StainsFile
 |

