Chemistry:Neighbouring group participation
In organic chemistry, neighbouring group participation (NGP, also known as anchimeric assistance) has been defined by the International Union of Pure and Applied Chemistry (IUPAC) as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma or pi bond contained within the parent molecule but not conjugated with the reaction centre.[1][1][2][3] When NGP is in operation it is normal for the reaction rate to be increased. It is also possible for the stereochemistry of the reaction to be abnormal (or unexpected) when compared with a normal reaction. While it is possible for neighbouring groups to influence many reactions in organic chemistry (e.g. the reaction of a diene such as 1,3-cyclohexadiene with maleic anhydride normally gives the endo isomer because of a secondary effect {overlap of the carbonyl group π orbitals with the transition state in the Diels-Alder reaction}) this page is limited to neighbouring group effects seen with carbocations and SN2 reactions.
NGP by heteroatom lone pairs
In this type of substitution reaction, one group of the substrate participates initially in the reaction and thereby affects the reaction. Due to NGP, the reaction rate gets increased by many folds. [clarification needed] A classic example of NGP is the reaction of a sulfur or nitrogen mustard with a nucleophile, the rate of reaction is much higher for the sulfur mustard and a nucleophile than it would be for a primary or secondary alkyl chloride without a heteroatom.[citation needed]
center|400px
NGP by an alkene
The π orbitals of an alkene can stabilize a transition state by helping to delocalize the positive charge of the carbocation. For instance the unsaturated tosylate will react more quickly (1011 times faster for aqueous solvolysis) with a nucleophile than the saturated tosylate.
center|250px
The carbocationic intermediate will be stabilized by resonance where the positive charge is spread over several atoms. In the diagram below this is shown.
center|600px
Here is a different view of the same intermediates. center|400px
Even if the alkene is more remote from the reacting center the alkene can still act in this way. For instance in the following alkyl benzenesulfonate the alkene is able to delocalise the carbocation.
center|650px
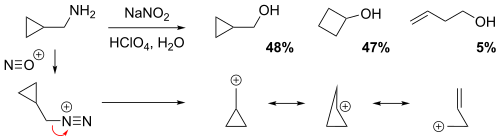
NGP by a cyclopropane, cyclobutane or a homoallyl group
The reaction of cyclopropylmethamine with sodium nitrite in dilute aqueous perchloric acid solution yielded a mixture of 48% cyclopropylmethyl alcohol, 47% cyclobutanol, and 5% homoallylic alcohol (but-3-en-1-ol).[4] In the non-classical perspective, the positive charge is delocalized throughout the carbocation intermediate structure via resonance, resulting in partial (electron-deficient) bonds. Evidently, the relatively low yield of the homoallylic alcohol implies that the homoallylic structure is the weakest resonance contributor.
NGP by an aromatic ring
An aromatic ring can assist in the formation of a carbocationic intermediate called a phenonium ion by delocalising the positive charge.
center|650px
When the following tosylate reacts with acetic acid in solvolysis then rather than a simple SN2 reaction forming B, a 48:48:4 mixture of A, B (which are enantiomers) and C+D was obtained [2] [3]. center|600px
The mechanism which forms A and B is shown below.
center|600px
NGP by aliphatic C-C or C-H bonds
Aliphatic C-C or C-H bonds can lead to charge delocalization if these bonds are close and antiperiplanar to the leaving group. Corresponding intermediates are referred to a nonclassical ions, with the 2-norbornyl system as the most well known case.
External links
References
- ↑ de Rooij, J. F.; Wille-Hazeleger, G.; Burgers, P. M.; van Boom, J. H. (1979). "Neighbouring group participation in the unblocking of phosphotriesters of nucleic acids". Nucleic Acids Research 6 (6): 2237–2259. doi:10.1093/nar/6.6.2237. PMID 461188.
- ↑ Stalford, Susanne A.; Kilner, Colin A.; Leach, Andrew G.; Turnbull, W. Bruce (2009-12-07). "Neighbouring group participation vs. addition to oxacarbenium ions: studies on the synthesis of mycobacterial oligosaccharides" (in en). Organic & Biomolecular Chemistry (Royal Society of Chemistry) 7 (23): 4842–4852. doi:10.1039/B914417J. PMID 19907773.
- ↑ Bowden, Keith (1993-01-01). "Neighbouring Group Participation by Carbonyl Groups in Ester Hydrolysis". in Bethell, D.. Advances in Physical Organic Chemistry Volume 28. Advances in Physical Organic Chemistry. 28. Academic Press, ScienceDirect. pp. 171–206. doi:10.1016/S0065-3160(08)60182-3. ISBN 9780120335282. http://www.sciencedirect.com/science/article/pii/S0065316008601823. Retrieved 2019-12-17.
- ↑ Roberts, J. D.; Mazur, R. H. (1951). "Small-Ring Compounds. IV. Interconversion Reactions of Cyclobutyl, Cyclopropylcarbinyl and Allylcarbinyl Derivatives". Journal of the American Chemical Society 73 (6): 2509–2520. doi:10.1021/ja01150a029.
- ^ Advanced organic chemistry, page 314, Jerry March (4th Ed), Wiley-Interscience.
- ^ Studies in Stereochemistry. I. The Stereospecific Wagner-Meerwein rearrangement of the Isomers of 3-Phenyl-2-butanol Donald J. Cram J. Am. Chem. Soc.; 1949; 71(12); 3863-3870. Abstract
- ^ Studies in Stereochemistry. V. Phenonium Sulfonate Ion-pairs as Intermediates in the Intramolecular Rearrangements and Solvolysis Reactions that Occur in the 3-Phenyl-2-butanol System Donald J. Cram J. Am. Chem. Soc.; 1952; 74(9); 2129-2137 Abstract.
 |