Chemistry:Sodium nitrite
| |||
| |||
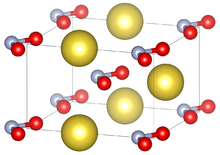 Unit cell of sodium nitrite under standard conditions
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium nitrite
| |||
| Preferred IUPAC name
Sodium nitrite | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1500 3287 | ||
| |||
| |||
| Properties | |||
| NaNO 2 | |||
| Molar mass | 68.9953 g/mol | ||
| Appearance | white or slightly yellowish crystalline solid | ||
| Density | 2.168 g/cm3 | ||
| Melting point | 271 °C (520 °F; 544 K) (decomposes at 320 °C) | ||
| |||
| Solubility in methanol | 4.4 g/(100 mL) | ||
| Solubility in ethanol | Soluble | ||
| Solubility in diethyl ether | 0.3 g/(100 mL) | ||
| Solubility in ammonia | Very soluble | ||
| Acidity (pKa) | ~9 | ||
| −14.5·10−6 cm3/mol | |||
Refractive index (nD)
|
1.65 | ||
| Structure[1] | |||
| Orthorhombic | |||
| Im2m | |||
a = 3.5653(8) Å, b = 5.5728(7) Å, c = 5.3846(13) Å
| |||
Formula units (Z)
|
2 | ||
| Thermochemistry | |||
Std molar
entropy (S |
106 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−359 kJ/mol[2] | ||
Gibbs free energy (ΔfG˚)
|
−295 kJ/mol | ||
| Pharmacology | |||
| 1=ATC code }} | V03AB08 (WHO) | ||
| Hazards | |||
| Safety data sheet | "Sodium nitrite". Safety Data Sheet. Sigma-Aldrich. 28 December 2022. https://www.sigmaaldrich.com/DE/en/sds/aldrich/563218. | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H272, H301, H319, H400 | |||
| P220, P273, P301+310, P305+351+338 | |||
| NFPA 704 (fire diamond) | |||
| 489 °C (912 °F; 762 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
180 mg/kg (rats, oral) | ||
| Related compounds | |||
Other anions
|
Sodium nitrate | ||
Other cations
|
|||
Related compounds
|
Nitrous acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
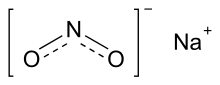
Sodium nitrite is an inorganic compound with the chemical formula NaNO
2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.[4]
Uses
Industrial chemistry
The main use of sodium nitrite is for the industrial production of organonitrogen compounds. It is a reagent for conversion of amines into diazo compounds, which are key precursors to many dyes, such as diazo dyes. Nitroso compounds are produced from nitrites. These are used in the rubber industry.[4]
It is used in a variety of metallurgical applications, for phosphatizing and detinning.[4]
Sodium nitrite is an effective corrosion inhibitor and is used as an additive in industrial greases,[5] as an aqueous solution in closed loop cooling systems, and in a molten state as a heat transfer medium.[6]
Food additive and preservative
Sodium nitrite is used to speed up the curing of meat,[7] inhibit the germination of Clostridium botulinum spores, and also impart an attractive pink color.[8][9] Nitrite reacts with the meat myoglobin to cause color changes, first converting to nitrosomyoglobin (bright red), then, on heating, to nitrosohemochrome (a pink pigment).[10]
Historically, salt has been used for the preservation of meat. The salt-preserved meat product was usually brownish-gray in color. When sodium nitrite is added with the salt, the meat develops a red, then pink color, which is associated with cured meats such as ham, bacon, hot dogs, and bologna.[11]
In the early 1900s, irregular curing was commonplace. This led to further research surrounding the use of sodium nitrite as an additive in food, standardizing the amount present in foods to minimize the amount needed while maximizing its food additive role.[12] Through this research, sodium nitrite has been found to give taste and color to the meat and inhibit lipid oxidation that leads to rancidity, with varying degrees of effectiveness for controlling growth of disease-causing microorganisms.[12] The ability of sodium nitrite to address the above-mentioned issues has led to production of meat with extended storage life and has improved desirable color and taste. According to scientists working for the meat industry,[13] nitrite has improved food safety.[12] This view is disputed in the light of the possible carcinogenic effects caused by adding nitrites to meat.[7]
Nitrite has the E number E250. Potassium nitrite (E249) is used in the same way. It is approved for usage in the European Union,[14][15] USA,[16] and Australia and New Zealand.[17]
In meat processing, sodium nitrite is never used in a pure state but always mixed with common salt. This mixture is known as nitrited salt, curing salt or nitrited curing salt. In Europe, nitrited curing salt contains between 99.1% and 99.5% common salt and between 0.5% and 0.9% nitrite. In the US, nitrited curing salt is dosed at 6.25%[18] and must be remixed with salt before use.[19]
Color and taste
The appearance and taste of meat is an important component of consumer acceptance.[12] Sodium nitrite is responsible for the desirable red color (or shaded pink) of meat.[12] Very little nitrite is needed to induce this change.[12] It has been reported that as little as 2 to 14 parts per million (ppm) is needed to induce this desirable color change.[20] However, to extend the lifespan of this color change, significantly higher levels are needed.[20] The mechanism responsible for this color change is the formation of nitrosylating agents by nitrite, which has the ability to transfer nitric oxide that subsequently reacts with myoglobin to produce the cured meat color.[20] The unique taste associated with cured meat is also affected by the addition of sodium nitrite.[12] However, the mechanism underlying this change in taste is still not fully understood.[20]
Inhibition of microbial pathogens
In conjunction with salt and pH levels, sodium nitrite reduces the ability of Clostridium botulinum spores to grow to the point of producing toxin.[9][21] Some dry-cured meat products are manufactured without nitrites. For example, Parma ham, which has been produced without nitrite since 1993, was reported in 2018 to have caused no cases of botulism. This is because the interior of the muscle is sterile and the surface is exposed to oxygen.[7] Other manufacture processes do not assure these conditions, and reduction of nitrite results in toxin production.[22]
Sodium nitrite has shown varying degrees of effectiveness for controlling growth of other spoilage or disease causing microorganisms.[12] Although the inhibitory mechanisms are not well known, its effectiveness depends on several factors including residual nitrite level, pH, salt concentration, reductants present and iron content.[20] The type of bacteria also affects sodium nitrite's effectiveness.[20] It is generally agreed that sodium nitrite is not effective for controlling Gram-negative enteric pathogens such as Salmonella and Escherichia coli.[20]
Other food additives (such as lactate and sorbate) provide similar protection against bacteria, but do not provide the desired pink color.[23][24][25][26]
Inhibition of lipid peroxidation
Sodium nitrite is also able to effectively delay the development of oxidative rancidity.[20] Lipid peroxidation is considered to be a major reason for the deterioration of quality of meat products (rancidity and unappetizing flavors).[20] Sodium nitrite acts as an antioxidant in a mechanism similar to the one responsible for the coloring effect.[20] Nitrite reacts with heme proteins and metal ions, neutralizing free radicals by nitric oxide (one of its byproducts).[20] Neutralization of these free radicals terminates the cycle of lipid oxidation that leads to rancidity.[20]
Medication
 Chemical structure | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | FDA Professional Drug Information |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | NNaO2 |
| Molar mass | 68.995 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sodium nitrite is used as a medication together with sodium thiosulfate to treat cyanide poisoning.[29] It is recommended only in severe cases of cyanide poisoning and has largely been replaced by use of hydroxocobalamin,[30] a form of vitamin B12, but given in much higher doses than needed nutritionally.[31] In those who have both cyanide poisoning and carbon monoxide poisoning sodium thiosulfate by itself is usually recommended if the facility does not have sufficient hydroxycobalamin.[32][33] It is given by slow injection into a vein.[29]
NaNO
2 side effects are chiefly related to creation of methemoglobinemia and vasodilation. Side effects can include low blood pressure, headache, shortness of breath, loss of consciousness, and vomiting.[29] Greater care should be taken in people with underlying heart disease.[29] The patient's levels of methemoglobin should be regularly checked during treatment.[29] While not well studied during pregnancy, there is some evidence of potential harm to the baby.[34] Sodium nitrite works by creating methemoglobin, where the iron atom at the center of the heme group is in the oxidized ferric (Fe3+) state, which binds with cyanide with greater affinity than its binding to the cytochrome C oxidase, and thus removes it from blocking the metabolic function of mitochondria.[34]
Sodium nitrite came into medical use in the 1920s and 1930s.[35][36] It is on the World Health Organization's List of Essential Medicines.[37]
Suicide
Several academic publications in 2020 and 2021 have discussed the toxicity of sodium nitrite, and an apparent recent increase in suicides using sodium nitrite which had been ordered online.[38] The usage of sodium nitrite as a suicide method has been heavily discussed on suicide forums, primarily Sanctioned Suicide.[39]
Sodium nitrite was also the focal-point of the McCarthy et al. v Amazon lawsuit alleging that Amazon knowingly assisted in the deaths of healthy children by selling them "suicide kits" as Amazon's "frequently bought together" feature recommended buying sodium nitrite, an antiemetic, and a suicide instruction book together.[40] This lawsuit was dismissed in June 2023.[41] The online marketplace eBay has globally prohibited the sale of sodium nitrite since 2019.[42] Kenneth Law, a Canadian distributor of sodium nitrite was prosecuted in 2023 for assisting suicide.[43][44] That same year, legislation was introduced in the United States with the aim of deeming sodium nitrite products with a sodium nitrite concentration of greater than 10% by volume to be banned consumer products under the Consumer Product Safety Act.[45]
In cases of suspected suicide involving sodium nitrite, it is critical that responding individuals administer immediate intravenous methylene blue.[46][47][48] Methylene blue is the antidote to the methemoglobinemia caused by intentional ingestion of sodium nitrite as a suicide agent.[49]
Toxicity
Sodium nitrite is toxic.[50] The LD50 in rats is 180 mg/kg and in humans LDLo is 71 mg/kg.[51] The mechanism by which sodium nitrite causes death is methemoglobinemia.[52] The oftentimes severe methemoglobinemia found in sodium nitrite poisoning cases results in systemic hypoxia, metabolic acidosis, and cyanosis.[53] The reported[54] signs of sodium nitrite poisoning are as follows:
Symptoms of [nitrite] poisoning can vary depending on the amount and duration of the exposure. Those with very mild methemoglobinemia might not have any symptoms at all, or might appear a little pale and feel tired. Moderate-to-severe poisoning is associated with cyanosis (blueness of the skin), confusion, loss of consciousness, seizures, abnormal heart rhythms, and death.
With prompt action, sodium nitrite poisoning is reversible using an antidote, methylene blue.[47] It has been reported[55] that sodium nitrite poisoning can also be detected post-mortem:
Postmortem detection of [methemoglobinemia] is typically established via screening techniques such as scene evidence suggesting fatal consumption of a toxic salt in addition to the characteristic grey-purple lividity observed upon the body. The diagnosis can be established via postmortem blood testing demonstrating elevated methemoglobin saturation. Additionally, we have confirmed that postmortem MRI in cases of [methemoglobinemia] demonstrates a T1-bright (hyperintense) signal of the blood; both within intracardiac blood on chest MRIs and postmortem blood samples in tubes.
Death by sodium nitrite ingestion can happen at lower doses than the previously known LDLo.[56][57] Sodium nitrite has been used for homicide[58][59] and suicide.[60][61] To prevent accidental intoxication, sodium nitrite (blended with salt) sold as a food additive in the US is dyed bright pink to avoid mistaking it for plain salt or sugar. In other countries, nitrited curing salt is not dyed but is strictly regulated.[62]
Occurrence in vegetables
Nitrites do not occur naturally in vegetables in significant quantities,[63] but deliberate fermentation of celery juice, for instance, with a naturally high level of nitrates, can produce nitrite levels sufficient for commercial meat curing.[64] Boiling vegetables does not affect nitrite levels.[65]
The presence of nitrite in animal tissue is a consequence of metabolism of nitric oxide, an important neurotransmitter.[66] Nitric oxide can be created de novo from nitric oxide synthase utilizing arginine or from ingested nitrite.[67]
Pigs
Due to sodium nitrite's high level of toxicity to swine (Sus scrofa) it is now being developed in Australia to control feral pigs and wild boar.[68][69] The sodium nitrite induces methemoglobinemia in swine, i.e. it reduces the amount of oxygen that is released from hemoglobin, so the animal will feel faint and pass out, and then die in a humane manner after first being rendered unconscious.[70] The Texas Parks and Wildlife Department operates a research facility at Kerr Wildlife Management Area, where they examine feral pig feeding preferences and bait tactics to administer sodium nitrite.[71]
Cancer
Carcinogenicity is the ability or tendency of a chemical to induce tumors, increase their incidence or malignancy, or shorten the time of tumor occurrence.[72]
Adding nitrites to meat has been shown to generate known carcinogens such as nitrosamines; the World Health Organization (WHO) advises that 50 g (1.8 oz) of "processed meats" a day would raise the risk of getting bowel cancer by 18% over a lifetime, and eating larger amounts raises the risk more. The World Health Organization's review of more than 400 studies concluded, in 2015, that there was sufficient evidence that "processed meats" caused cancer, particularly colon cancer;[7] the WHO's International Agency for Research on Cancer (IARC) classified "processed meats" as carcinogenic to humans (Group 1); "processed meat" meaning meat that has been transformed through salting, curing, fermentation, smoking, or other processes to enhance flavour or improve preservation.).[7][73]
Nitrosamines can be formed during the curing process used to preserve meats, when sodium nitrite-treated meat is cooked, and also from the reaction of nitrite with secondary amines under acidic conditions (such as occurs in the human stomach). Dietary sources of nitrosamines include US cured meats preserved with sodium nitrite as well as the dried salted fish eaten in Japan. In the 1920s, a significant change in US meat curing practices resulted in a 69% decrease in average nitrite content. This event preceded the beginning of a dramatic decline in gastric cancer mortality.[74] Around 1970, it was found that ascorbic acid (vitamin C), an antioxidant, inhibits nitrosamine formation.[75] Consequently, the addition of at least 550 ppm of ascorbic acid is required in meats manufactured in the United States. Manufacturers sometimes instead use erythorbic acid, a cheaper but equally effective isomer of ascorbic acid. Additionally, manufacturers may include α-tocopherol (vitamin E) to further inhibit nitrosamine production. α-Tocopherol, ascorbic acid, and erythorbic acid all inhibit nitrosamine production by their oxidation-reduction properties. Ascorbic acid, for example, forms dehydroascorbic acid when oxidized, which when in the presence of nitrosonium, a potent nitrosating agent formed from sodium nitrite, reduces the nitrosonium into nitric oxide.[76] The nitrosonium ion formed in acidic nitrite solutions is commonly[77][78] mislabeled nitrous anhydride, an unstable nitrogen oxide that cannot exist in vitro.[79]
Ingesting nitrite under conditions that result in endogenous nitrosation has been classified as "probably carcinogenic to humans" by International Agency for Research on Cancer (IARC).[80][81]
Sodium nitrite consumption has also been linked to the triggering of migraines in individuals who already experience them.[82]
One study has found a correlation between highly frequent ingestion of meats cured with pink salt and the COPD form of lung disease.[83][84] The study's researchers suggest that the high amount of nitrites in the meats was responsible; however, the team did not prove the nitrite theory. Additionally, the study does not prove that nitrites or cured meat caused higher rates of COPD, merely a link. The researchers did adjust for many of COPD's risk factors, but they commented they cannot rule out all possible unmeasurable causes or risks for COPD.[85][86]
Production
Industrial production of sodium nitrite follows one of two processes, the reduction of nitrate salts, or the oxidation of lower nitrogen oxides.
One method uses molten sodium nitrate as the salt, and lead which is oxidized, while a more modern method uses scrap iron filings to reduce the nitrate.[4][87]
- NaNO
3 + Pb → NaNO
2 + PbO - NO−
3 + Fe + 2H+
→ NO−
2 + Fe2+
+ H
2O
A more commonly used method involves the general reaction of nitrogen oxides in alkaline aqueous solution, with the addition of a catalyst. The exact conditions depend on which nitrogen oxides are used, and what the oxidant is, as the conditions need to be carefully controlled to avoid over oxidation of the nitrogen atom.[4]
- 2 NaOH + NO
2 + NO → 2 NaNO
2 + H
2O - 2 NaOH + N
2O
3 → 2 NaNO
2 + H
2O
Sodium nitrite has also been produced by reduction of nitrate salts by exposure to heat, light, ionizing radiation, metals, hydrogen, and electrolytic reduction.[88]
- NaNO
3 + CaSO
3 → NaNO
2 + CaSO
4
Chemical reactions
In the laboratory, sodium nitrite can be used to destroy excess sodium azide.[89][90]
- 2 NaN
3 + 2 NaNO
2 + 4 H+
→ 3 N
2 + 2 NO + 4 Na+
+ 2 H
2O
Above 330 °C sodium nitrite decomposes (in air) to sodium oxide, nitric oxide and nitrogen dioxide.[91]
- 2 NaNO
2 → Na
2O + NO + NO
2
Sodium nitrite can also be used in the production of nitrous acid:
- 2 NaNO
2 + H
2SO
4 → 2 HNO
2 + Na
2SO
4
The nitrous acid then, under normal conditions, decomposes:
- 2 HNO
2 → NO
2 + NO + H
2O
The resulting nitrogen dioxide hydrolyzes to a mixture of nitric and nitrous acids:
- 2 NO
2 + H
2O → HNO
3 + HNO
2
Isotope labelling 15N

In organic synthesis isotope enriched sodium nitrite-15N can be used instead of normal sodium nitrite as their reactivity is nearly identical in most reactions.
The obtained products carry isotope 15N and hence nitrogen NMR can be efficiently carried out.[92]
References
- ↑ "The Refinement of the Structure of Ferroelectric Sodium Nitrite". Journal of the Korean Physical Society 29: 551–554. November 1996.
- ↑ Chemical Principles (6th ed.). Houghton Mifflin Company. 2009. p. A23. ISBN 978-0-618-94690-7.
- ↑ "GESTIS-Stoffdatenbank sodium nitrite". https://gestis.dguv.de/data?name=001380&lang=en.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Nitrates and Nitrites". Ullmann's Encyclopedia of Industrial Chemistry. 2006. doi:10.1002/14356007.a17_265. ISBN 978-3-527-30673-2.
- ↑ "Determination of sodium nitrite in complex sodium oils". Chemistry and Technology of Fuels and Oils 20 (12): 612–613. 1984. doi:10.1007/BF00726438.
- ↑ "Sodium Nitrite". General Chemical. http://www.generalchemical.com/sodium-nitrite.html.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Yes, bacon really is killing us" (in en-GB). The Guardian (London). 1 March 2018. ISSN 0261-3077. https://www.theguardian.com/news/2018/mar/01/bacon-cancer-processed-meats-nitrates-nitrites-sausages. "The firms who sold nitrite powders to ham-makers spoke quite openly about how the main advantage was to increase profit margins by speeding up production."
- ↑ "Use of sodium nitrite in salt-curing of Atlantic salmon (Salmo salar L.) – Impact on product quality". Food Chemistry 124 (3): 759–766. February 2011. doi:10.1016/j.foodchem.2010.06.092.
- ↑ 9.0 9.1 "Use and Removal of Nitrite in Meat Products". https://www.fsai.ie/faq/use_and_removal_of_nitrite.html#prevent_botulism.
- ↑ "Cured Meat Pigments, Studies of the Photooxidation of Nitrosomyoglobin". Journal of Agricultural and Food Chemistry 12 (1): 89–93. January 1964. doi:10.1021/jf60131a026. Bibcode: 1964JAFC...12...89B.
- ↑ ""Meat Pigment Chemistry", taken from IFT Mini-Experiments in Food Science Series". http://www.math.unl.edu/~jump/Center1/Labs/MeatPigmentChemistry.pdf.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 "Human safety controversies surrounding nitrate and nitrite in the diet". Nitric Oxide 26 (4): 259–266. May 2012. doi:10.1016/j.niox.2012.03.011. PMID 22487433.
- ↑ "Science Says: Are hot dogs healthier without added nitrites? | Lifestyle from CTV News". 30 June 2017. https://www.ctvnews.ca/lifestyle/article/science-says-are-hot-dogs-healthier-without-added-nitrites/.
- ↑ "Approved additives and E numbers" (in en). https://www.food.gov.uk/business-guidance/approved-additives-and-e-numbers.
- ↑ "Opinions of the Scientific Committee for Food on: Nitrates and Nitrite". Food Science and Techniques. European Commission. http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_38.pdf.
- ↑ US Food and Drug Administration: "Listing of Food Additives Status Part II". https://www.fda.gov/Food/FoodIngredientsPackaging/FoodAdditives/ucm191033.htm#ftnT.
- ↑ "Australia New Zealand Food Standards Code - Standard 1.2.4 - Labelling of Ingredients" (in en). http://www.legislation.gov.au/Details/F2011C00827/Html/Text.
- ↑ "Sausages and Cured Foods". Professional Cooking, College Version. Hoboken, New Jersey: John Wiley & Sons. 2006. p. 827. ISBN 978-0-471-66374-4. https://books.google.com/books?id=hxcH5o9QeywC&pg=PA827. Retrieved 2013-08-16.
- ↑ Who poisoned your bacon?. Icon Books. February 2021. pp. xv. ISBN 978-1-78578-611-2. https://iconbooks.com/ib-title/who-poisoned-your-bacon/.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 "Sodium Nitrite in Processed Meat and Poultry Meats: A Review of Curing and Examining the Risk/Benefit of Its Use". American Meat Science Association 3: 1–14. November 2011. http://www.meatscience.org/docs/default-source/publications-resources/white-papers/2011-11-amsa-nitrite-white-paper.pdf?sfvrsn=8.
- ↑ "Microbiological safety of processed meat products formulated with low nitrite concentration - A review". Asian-Australasian Journal of Animal Sciences 31 (8): 1073–1077. August 2018. doi:10.5713/ajas.17.0675. PMID 29531192.
- ↑ "Influence of reduced levels or suppression of sodium nitrite on the outgrowth and toxinogenesis of psychrotrophic Clostridium botulinum Group II type B in cooked ham". International Journal of Food Microbiology 334. December 2020. doi:10.1016/j.ijfoodmicro.2020.108853. PMID 32932195.
- ↑ "Effects of potassium sorbate and other antibotulinal agents on germination and outgrowth of Clostridium botulinum type E spores in microcultures". Applied and Environmental Microbiology 44 (5): 1212–1221. November 1982. doi:10.1128/AEM.44.5.1212-1221.1982. PMID 6758699. Bibcode: 1982ApEnM..44.1212S.
- ↑ "Effects of various concentrations of sodium nitrite and potassium sorbate on Clostridium botulinum toxin production in commercially prepared bacon". Journal of Food Science 45 (5): 1285–1292. September 1980. doi:10.1111/j.1365-2621.1980.tb06539.x.
- ↑ "The control of Clostridium botulinum during extended storage of pressure-treated, cooked chicken". Food Control 37: 104–108. March 2014. doi:10.1016/j.foodcont.2013.09.042.
- ↑ "The inhibitory effects of sorbate and benzoate against Clostridium perfringens type A isolates". Food Microbiology 48: 89–98. June 2015. doi:10.1016/j.fm.2014.12.007. PMID 25790996.
- ↑ "Therapeutic goods exempted from pregnancy categorisation | Therapeutic Goods Administration (TGA)". https://www.tga.gov.au/therapeutic-goods-exempted-pregnancy-categorisation.
- ↑ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". 21 June 2022. https://www.tga.gov.au/resources/publication/publications/prescription-medicines-registration-new-generic-medicines-and-biosimilar-medicines-2017.
- ↑ 29.0 29.1 29.2 29.3 29.4 WHO Model Formulary 2008. World Health Organization. 2009. p. 65. ISBN 978-92-4-154765-9.
- ↑ "Sodium Nitrite Solution for Injection - Summary of Product Characteristics (SPC) - (eMC)". https://www.medicines.org.uk/emc/medicine/32282.
- ↑ "Patterns of cyanide antidote use since regulatory approval of hydroxocobalamin in the United States". American Journal of Therapeutics 21 (4): 244–249. July–August 2014. doi:10.1097/MJT.0b013e31824ea656. PMID 23689094.
- ↑ "Cyanide Toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. 2023. https://www.ncbi.nlm.nih.gov/books/NBK507796/. Retrieved 7 December 2023.
- ↑ (in en) Pediatric Emergency Medicine. Elsevier Health Sciences. 2008. p. 1018. ISBN 978-1-4160-0087-7. https://books.google.com/books?id=wpvux6RS-jsC&pg=PA1018.
- ↑ 34.0 34.1 "Sodium Nitrite Injection - FDA prescribing information, side effects and uses". https://www.drugs.com/pro/sodium-nitrite-injection.html.
- ↑ (in en) Medical Toxicology. Lippincott Williams & Wilkins. 2004. p. 172. ISBN 978-0-7817-2845-4. https://books.google.com/books?id=BfdighlyGiwC&pg=PA172.
- ↑ (in en) Nitrite and Nitrate in Human Health and Disease. Springer Science & Business Media. 2011. p. 226. ISBN 978-1-60761-616-0. https://books.google.com/books?id=CDkkwVV4aMQC&pg=PA226.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Durão C, et al., 2020.; Durão C, et al., 2021.; Tomsia M, et al., 2021.; McCann SD, Kennedy JM, et al., 2021.; Dean DE, et al., 2021.; Hickey TB, et al., 2021.; Harvey M, et al., 2010.; McCann SD, Tweet MS, Wahl MS, 2021.; Sedhai YR, et al., 2022.; Mudan A, et al., 2020.
- ↑ "Where the Despairing Log On, and Learn Ways to Die". The New York Times. 9 December 2021. https://www.nytimes.com/interactive/2021/12/09/us/where-the-despairing-log-on.html.
- ↑ "Amazon 'suicide kits' have led to teen deaths, according to new lawsuit". Ars Technica. 7 October 2022. https://arstechnica.com/tech-policy/2022/10/amazon-suicide-kits-have-led-to-teen-deaths-according-to-new-lawsuit/.
- ↑ "Judge dismisses lawsuit claiming Amazon sold 'suicide kits' to teenagers" (in en). Reuters. 28 June 2023. https://www.reuters.com/legal/judge-dismisses-lawsuit-claiming-amazon-sold-suicide-kits-teenagers-2023-06-28/.
- ↑ Director, eBay (UK) Limited (8 January 2021). "Re Jason Thompson (deceased) – Sodium Nitrite". https://www.judiciary.uk/wp-content/uploads/2020/12/2020-0246-Response-from-Ebay-UK-Ltd-Redacted.pdf.
- ↑ "Here's why the world will be watching Kenneth Law's court battle". CTV Toronto. 25 July 2023. https://www.ctvnews.ca/toronto/article/heres-why-the-world-will-be-watching-kenneth-laws-court-battle/.
- ↑ "88 UK deaths linked to Canada 'poison seller'". BBC News. 25 August 2023. https://www.bbc.com/news/world-us-canada-66609884.
- ↑ "Chemical Used in Suicides Faces Sales Ban Under Proposed Law" (in en). 22 June 2023. https://news.bloomberglaw.com/us-law-week/chemical-used-in-suicides-faces-sales-ban-under-proposed-law.
- ↑ "Nitrite intoxication: Protection with methylene blue and oxygen". Toxicology and Applied Pharmacology 30 (2): 221–226. 1 November 1974. doi:10.1016/0041-008X(74)90093-3. ISSN 0041-008X. Bibcode: 1974ToxAP..30..221S.
- ↑ 47.0 47.1 "Immediate methylene blue is critical for sodium nitrite ingestions". The American Journal of Emergency Medicine 68: 186. June 2023. doi:10.1016/j.ajem.2023.04.034. PMID 37120398.
- ↑ "2022 Health Advisory #22 Poisonings and Death Related to Intentional Sodium Nitrite Ingestions". 8 September 2022. https://www.nyc.gov/assets/doh/downloads/pdf/han/advisory/2022/sodium-nitrate-ingestion-poisonings.pdf.
- ↑ "Antidote for acquired methemoglobinemia: methylene blue". Journal of the Korean Medical Association 56 (12): 1084–1090. 16 December 2013. doi:10.5124/jkma.2013.56.12.1084. https://synapse.koreamed.org/articles/1042644.
- ↑ "How toxic is it?". https://www.ase.org.uk/sites/default/files/chemistry%20PDFs/PDFs/How%20toxic%20is%20it.pdf.
- ↑ "Safety data for sodium nitrite". The Physical and Theoretical Chemistry Laboratory. Oxford University. http://msds.chem.ox.ac.uk/SO/sodium_nitrite.html.
- ↑ "Severe Methemoglobinemia due to Sodium Nitrite Poisoning". Case Reports in Emergency Medicine 2016. 3 August 2016. doi:10.1155/2016/9013816. PMID 27563472.
- ↑ "Sodium Nitrite Intoxication and Death: Summarizing Evidence to Facilitate Diagnosis". International Journal of Environmental Research and Public Health 19 (21). October 2022. doi:10.3390/ijerph192113996. PMID 36360874.
- ↑ "Nitrate and Nitrite Poisoning" (in en). https://www.poison.org/articles/causes-and-symptoms-of-nitrate-nitrite-poisoning-174.
- ↑ "Fatal methemoglobinemia: A case series highlighting a new trend in intentional sodium nitrite or sodium nitrate ingestion as a method of suicide". Forensic Science International 326. September 2021. doi:10.1016/j.forsciint.2021.110907. PMID 34298207.
- ↑ "Fatal methaemoglobinaemia in a dental nurse. A case of sodium nitrite poisoning". The British Journal of General Practice 40 (340): 470–471. November 1990. PMID 2271282.
- ↑ "Death associated with nitrite ingestion: report of a case". Journal of Forensic Sciences 24 (4): 768–771. October 1979. doi:10.1520/JFS10905J. PMID 541641.
- ↑ "Chinese teacher sentenced to death for poisoning nursery children". BBC News. 29 September 2020. https://www.bbc.com/news/world-asia-china-54335841.
- ↑ "Teacher in China sentenced to death for poisoning children's porridge". The Guardian. Agence France-Presse in Beijing. 29 September 2020. https://www.theguardian.com/world/2020/sep/29/teacher-in-china-sentenced-to-death-for-poisoning-childrens-porridge.
- ↑ "'General Hospital' Actress Lindsey Pearlman's Cause of Death Revealed". The Hollywood Reporter. 16 August 2022. https://www.hollywoodreporter.com/news/general-news/lindsey-pearlman-suicide-coroners-office-1235200099/.
- ↑ "Police charge Mississauga man in connection with sale, distribution of sodium nitrite in GTA". CTV News. 3 May 2023. https://www.ctvnews.ca/toronto/article/police-charge-mississauga-man-in-connection-with-sale-distribution-of-sodium-nitrite-in-gta/.
- ↑ "The Use and Removal of Nitrite in Meat Products | FAQs". The Food Safety Authority of Ireland. https://www.fsai.ie/faq/use_and_removal_of_nitrite.html.
- ↑ "Nitrates and Nitrites". Encyclopedia of Food Sciences and Nutrition. 2003. pp. 4136–4141. doi:10.1016/B0-12-227055-X/00830-0. ISBN 978-0-12-227055-0.
- ↑ "Is celery juice a viable alternative to nitrites in cured meats?" (in en). https://www.mcgill.ca/oss/article/food/celery-juice-viable-alternative-nitrites-cured-meats.
- ↑ "Effects of some processing methods on nitrate and nitrite changes in cruciferous vegetables". Journal of Food Composition and Analysis 22 (4): 315–321. June 2009. doi:10.1016/j.jfca.2008.10.025.
- ↑ "Measurement of nitrite and nitrate levels in biological samples by capillary electrophoresis". Journal of Chromatography. B, Biomedical Applications 660 (2): 401–404. October 1994. doi:10.1016/0378-4347(94)00310-6. PMID 7866533.
- ↑ "Nitrogen oxides and hydroxyguanidines: formation of donors of nitric and nitrous oxides and possible relevance to nitrous oxide formation by nitric oxide synthase". Nitric Oxide 2 (4): 270–286. August 1998. doi:10.1006/niox.1998.0187. PMID 9851368.
- ↑ "Is America Ready for a Humane Feral Pig Toxicant?". Wildlife Damage Management Conference. Saratoga Springs, NY. 4 May 2009. https://digitalcommons.usu.edu/wdmconference/2009/session2/2/.
- ↑ Cowled BD, Lapidge SJ, Humphrys S, Staples L, "Nitrite Salts as Poisons in Baits for Omnivores", WO patent 2008/104028, published 2008
- ↑ Assessing the humaness and efficacy of a new feral pig bait in domestic pigs. Study PC0409. Canberra, South Australia: Veterinary Services Division, Institute of Medical and Veterinary Science. 2010. p. 11. http://www.environment.gov.au/biodiversity/invasive/publications/pubs/pigs-imvs-report.pdf.
- ↑ "Hogs Wild – Fighting the Feral Pig Problem". Texas Parks and Wildlife. 21 February 2013. https://www.youtube.com/watch?v=GigpxLNbgeg.
- ↑ "Known and Probable Human Carcinogens" (in en). https://www.cancer.org/cancer/cancer-causes/general-info/known-and-probable-human-carcinogens.html.
- ↑ "IARC Monographs evaluate consumption of red meat and processed meat". International Agency for Research on Cancer. 26 October 2015. https://www.iarc.who.int/wp-content/uploads/2018/07/pr240_E.pdf. "Processed meat was classified as carcinogenic to humans (Group 1), based on sufficient evidence in humans that the consumption of processed meat causes colorectal cancer."
- ↑ "The epidemiological enigma of gastric cancer rates in the US: was grandmother's sausage the cause?". International Journal of Epidemiology 30 (1): 181–182. February 2001. doi:10.1093/ije/30.1.181. PMID 11171883.
- ↑ "The inhibition of bacterially mediated N-nitrosation by vitamin C: relevance to the inhibition of endogenous N-nitrosation in the achlorhydric stomach". Carcinogenesis 10 (2): 397–399. February 1989. doi:10.1093/carcin/10.2.397. PMID 2492212.
- ↑ "Research Newsletter". 1 July 2014. http://lpi.oregonstate.edu/publications/research-newsletter.
- ↑ "Formation and occurrence of nitrosamines in food". Cancer Research 43 (5 Suppl): 2435s–2440s. May 1983. NAID 80001710206. PMID 6831466.
- ↑ Handbook of Food Analysis (Third ed.). CRC Press. 2015. p. 290. ISBN 978-1-4822-9784-3.
- ↑ "Reagents effecting nitrosation". Nitrosation Reactions and the Chemistry of Nitric Oxide. 2004. pp. 1–34. doi:10.1016/B978-044451721-0/50002-5. ISBN 978-0-444-51721-0.
- ↑ "List of classifications, Volumes 1–116 – IARC Monographs on the Evaluation of Carcinogenic Risks to Humans". International Agency for Research on Cancer (IARC) – World Health Organization (WHO). 2010. http://monographs.iarc.fr/ENG/Classification/latest_classif.php.
- ↑ Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 94. International Agency for Research on Cancer (IARC) – World Health Organization (WHO). 2010. ISBN 978-92-832-1294-2. http://monographs.iarc.fr/ENG/Monographs/vol94/index.php. Retrieved 25 September 2016.
- ↑ "Heading Off Migraine Pain". FDA Consumer magazine. U.S. Food and Drug Administration. 1998. http://permanent.access.gpo.gov/lps1609/www.fda.gov/fdac/features/1998/398_pain.html.
- ↑ "Study: Cured Meats, COPD May Be Linked". WebMD Medical News. 17 April 2007. http://www.webmd.com/news/20070417/study-copd-cured-meats-may-be-linked.
- ↑ "Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults". American Journal of Respiratory and Critical Care Medicine 175 (8): 798–804. April 2007. doi:10.1164/rccm.200607-969OC. PMID 17255565.
- ↑ "Study: Cured Meats, COPD May Be Linked". WebMD Medical News. 17 April 2007. http://www.webmd.com/news/20070417/study-copd-cured-meats-may-be-linked.
- ↑ "Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults". American Journal of Respiratory and Critical Care Medicine 175 (8): 798–804. April 2007. doi:10.1164/rccm.200607-969OC. PMID 17255565.
- ↑ "Reductive denitrification of nitrate by scrap iron filings". Journal of Zhejiang University. Science. B 6 (3): 182–186. March 2005. doi:10.1631/jzus.2005.B0182. PMID 15682502.
- ↑ "Sodium Nitrate and Nitrite". Kirk-Othmer Encyclopedia of Chemical Technology. 2006. doi:10.1002/0471238961.1915040916151115.a01.pub2. ISBN 978-0-471-23896-6.
- ↑ "Sodium Azide". Hazardous Waste Management. Northeastern University. March 2003. http://www.ehs.neu.edu/hazardous_waste/fact_sheets/sodium_azide/.
- ↑ Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C.: National Academy Press. 1995. doi:10.17226/4911. ISBN 978-0-309-05229-0. http://books.nap.edu/openbook.php?record_id=4911&page=165.
- ↑ "High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites". Journal of Physical and Chemical Reference Data 1 (3): 747–772. July 1972. doi:10.1063/1.3253104. Bibcode: 1972JPCRD...1..747S.
- ↑ "Molecular lambda shape light-driven dual switches: Spectroscopic and computational studies of the photoisomerization of bisazo Tröger base analogs". Journal of Molecular Structure 1178: 538–543. February 2019. doi:10.1016/j.molstruc.2018.10.071. Bibcode: 2019JMoSt1178..538K.
Sources
- "A fatal case by a suicide kit containing sodium nitrite ordered on the internet". Journal of Forensic and Legal Medicine 73. July 2020. doi:10.1016/j.jflm.2020.101989. PMID 32658747.
- "Another suicide by sodium nitrite and multiple drugs: an alarming trend for "exit"?". Forensic Science, Medicine, and Pathology 17 (2): 362–366. June 2021. doi:10.1007/s12024-020-00340-2. PMID 33247411.
- "Sodium nitrite detection in costal cartilage and vitreous humor - Case report of fatal poisoning with sodium nitrite". Journal of Forensic and Legal Medicine 81. July 2021. doi:10.1016/j.jflm.2021.102186. PMID 34058704.
- "Sodium Nitrite Ingestion: an Emerging Trend in Suicide Attempts Shared via Online Communities". The Journal of Emergency Medicine 60 (3): 409–412. March 2021. doi:10.1016/j.jemermed.2020.10.021. PMID 33712114.
- "Fatal methemoglobinemia in three suicidal sodium nitrite poisonings". Journal of Forensic Sciences 66 (4): 1570–1576. July 2021. doi:10.1111/1556-4029.14689. PMID 33598944.
- "Fatal methemoglobinemia: A case series highlighting a new trend in intentional sodium nitrite or sodium nitrate ingestion as a method of suicide". Forensic Science International 326. September 2021. doi:10.1016/j.forsciint.2021.110907. PMID 34298207.
- "Fatal methaemoglobinaemia induced by self-poisoning with sodium nitrite". Emergency Medicine Australasia 22 (5): 463–465. October 2010. doi:10.1111/j.1742-6723.2010.01335.x. PMID 21040485.
- "Rising incidence and high mortality in intentional sodium nitrite exposures reported to US poison centers". Clinical Toxicology 59 (12): 1264–1269. December 2021. doi:10.1080/15563650.2021.1905162. PMID 33787434.
- "The use of sodium nitrite for deliberate self-harm, and the online suicide market: Should we care?". The Medico-Legal Journal 90 (2): 79–80. June 2022. doi:10.1177/0025817221998119. PMID 33906496.
- "Severe Methemoglobinemia and Death From Intentional Sodium Nitrite Ingestions". The Journal of Emergency Medicine 59 (3): e85–e88. September 2020. doi:10.1016/j.jemermed.2020.06.031. PMID 32713620.
Further reading
- National Toxicology Program (May 2001). "Toxicology and carcinogenesis studies of sodium nitrite (CAS NO. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies)". National Toxicology Program Technical Report Series 495: 7–273. PMID 12563346. https://ntp.niehs.nih.gov/publications/reports/tr/tr495.
External links
- "Sodium nitrite". International Chemical Safety Card. IPCS INCHEM. http://www.inchem.org/documents/icsc/icsc/eics1120.htm.
- "Nitrite in Meat". University of Minnesota. 2011. http://www.extension.umn.edu/distribution/nutrition/DJ0974.html.
 |



