Chemistry:Nemertelline
From HandWiki
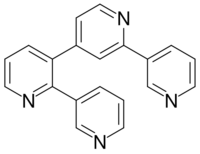
| |
| Names | |
|---|---|
| Preferred IUPAC name
13,22:23,34:32,43-Quaterpyridine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
| MeSH | Nemertelline |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H14N4 | |
| Molar mass | 310.360 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Nemertelline is a neurotoxic tetra-pyridine compound originally found in the marine ribbon worm Amphiporus angulatus.[1] These worms produce a variety of toxins which are used both in hunting their prey and in defending themselves from predators.[2] Interest in potential application of this compound as an antifouling agent for boats and other marine installations has led to attempts to produce it synthetically by convenient routes.[3] Its toxicity is similar to nicotine in crustaceans but has no mammalian toxicity. It is similar to nicotelline in structure.[4]
References
- ↑ "Hoplonemertine worms -- a new source of pyridine neurotoxins". Experientia 32 (6): 684–6. June 1976. doi:10.1007/BF01919831. PMID 181266.
- ↑ "Inhibition of barnacle larval settlement and crustacean toxicity of some hoplonemertine pyridyl alkaloids". Biomolecular Engineering 20 (4-6): 355–61. July 2003. doi:10.1016/S1389-0344(03)00049-2. PMID 12919820.
- ↑ "An efficient two-step total synthesis of the quaterpyridine nemertelline". The Journal of Organic Chemistry 68 (26): 10178–80. December 2003. doi:10.1021/jo034805b. PMID 14682721.
- ↑ "Hoplonemertine worms -- a new source of pyridine neurotoxins". Experientia 32 (6): 684–6. June 1976. doi:10.1007/BF01919831. PMID 181266.
 |
