Chemistry:Nicotelline
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3,2′:4′,3′′-Terpyridine
| |
| Other names
Nicotellin; 2,4-Dipyridin-3-ylpyridine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H11N3 | |
| Molar mass | 233.274 g·mol−1 |
| Melting point | 147–148 °C (297–298 °F; 420–421 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Nicotelline is an alkaloid first identified in 1914 as a chemical constituent of tobacco plants (Nicotiana).[2]
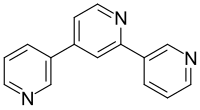
The chemical structure of nicotelline wasn't elucidated until 1956, when it was determined that nicotelline is a terpyridine consisting of three linked pyridine rings.[3] This structure was confirmed by laboratory synthesis.[3][4] Nicotelline has the molecular formula C
15H
11N
3. It is a crystalline solid with a melting point of 147-148 °C.[1] It is soluble in hot water, chloroform, ethanol, and benzene.[1]
Nicotelline has long been known to be a constituent of tobacco smoke.[5] As such, it has recently been proposed as a biomarker or environmental tracer for tobacco smoke.[6]
References
- ↑ 1.0 1.1 1.2 The Merck Index (12th ed.). p. 1119. 6609. Nicotelline.
- ↑ Noga, Eugen (1914). "The alkaloids in tobacco extract". Fach. Mitt. Tabakregie (1 and 2).
- ↑ 3.0 3.1 Kuffner, Friedrich; Kaiser, Ernst (1954). "Nicotellin and the synthesis of a new terpyridyl". Monatshefte für Chemie 85: 896–905. doi:10.1007/BF00898717.
- ↑ Kuffner, Friedrich; Faderl, Norbert (1956). "Constitution of nicotelline". Monatshefte für Chemie 87: 71–81. doi:10.1007/BF00903590.
- ↑ Kuffner, F. (1956). "The alkaloids of tobacco smoke and the constitution of nicotelline". Fachliche Mitteilungen der Oesterreichischen Tabakregie: 18–19.
- ↑ Jacob, Peyton; Goniewicz, Maciej L.; Havel, Christopher M.; Schick, Suzaynn F.; Benowitz, Neal L. (2013). "Nicotelline: A Proposed Biomarker and Environmental Tracer for Particulate Matter Derived from Tobacco Smoke". Chemical Research in Toxicology 26 (11): 1615–1631. doi:10.1021/tx400094y. PMID 24125094.
 |

