Chemistry:Neo-Inositol
| |
| Names | |
|---|---|
| IUPAC name
neo-Inositol[2]
| |
| Systematic IUPAC name
(1R,2R,3s,4S,5S,6s)-cyclohexane-1,2,3,4,5,6-hexol | |
| Other names
(1s,2R,3R,4s,5S,6S)-cyclohexane-1,2,3,4,5,6-hexol;
1,2,3/4,5,6-cyclohexanehexol[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| UNII | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
| Appearance | white crystalline solid [3] |
| Density | 1.697 g/ml (from X-ray structure)[4] |
| Melting point | 315 °C; 599 °F; 588 K[5][6] |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system and skin.[7] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
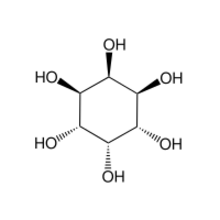
The chemical compound neo-inositol is one of the nine stereoisomers cyclohexane-1,2,3,4,5,6-hexol, the "inositols". Its formula is C
6H
12O
6; the six carbon atoms form a ring, each of them is bonded to a hydrogen atom and a hydroxyl group (–OH). If the ring is assumed horizontal, three consecutive hydroxyls lie above the respective hydrogens, and the other three lie below them.
Like the other stereoisomers, neo-inositol is considered a carbohydrate, specifically a sugar alcohol (to distinguish it from the more familiar ketose and aldose sugars, like glucose). It occurs in nature, but only in small amounts; usually much smaller than those of myo-inositol, the most important stereoisomer.[8]
Chemical and physical properties
Crystal structure
neo-inositol crystallizes in the triclinic system with group . The cell parameters are a = 479.9 pm, b = 652.0 pm, c = 650.5 pm, α = 70.61°, β = 69.41°, γ = 73.66°, Z = 1, with molecular symmetry . The cell volume is 0.176 nm3. The ring has the chair conformation with puckering parameter Q = 60.9 pm.[4]
Synthesis
neo-Inositol can be obtained from para-benzoquinone via conduritol intermediates.[9]
Natural occurrence and biological roles
Small amounts of neo-inositol can be deteceted in human urine.[10]
See also
- allo-Inositol
- cis-Inositol
- D-chiro-Inositol
- L-chiro-Inositol
- epi-Inositol
- muco-Inositol
- scyllo-Inositol
References
- ↑ "Neo-Inositol". http://www.ebi.ac.uk/chebi/searchId.do;jsessionid=B128111F38B12846027AAFB90CCC28A6?chebiId=25492. Retrieved 9 October 2012.
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 1415. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Synthose Inc. (2024): "[1]". Product catalog page at synthose.com. Accessed on 2024-07-02.
- ↑ 4.0 4.1 Younghee Yeon (2001): "The crystal and molecular structures of neo-inositol and two forms of scyllo-inositol". Korean Journal of Crystallography, volume 12, issue 3, pages 150-156.
- ↑ Alexandra Simperler, Stephen W. Watt, P. Arnaud Bonnet, William Jones, W. D. Samuel Motherwell (2006): "Correlation of melting points of inositols with hydrogen bonding patterns". CrystEngComm, volume 8, pages 589-600 doi:10.1039/B606107A
- ↑ Watt, S. W.; Chisholm, J. A.; Jones, W.; Motherwell, S. (2004). "A Molecular Dynamics Simulation of the Melting Points and Glass Transition Temperatures of Myo- and Neo-Inositol". Journal of Chemical Physics 121 (19): 9565–9573. doi:10.1063/1.1806792. PMID 15538878. Bibcode: 2004JChPh.121.9565W.
- ↑ "Material Safety Data Sheet". Sigma-Aldrich. http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=516163&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F516163%3Flang%3Den. Retrieved 9 October 2012.
- ↑ Michell, R. H. (February 2008). "Inositol Derivatives: Evolution and Functions". Nature Reviews Molecular Cell Biology 9 (2): 151–61. doi:10.1038/nrm2334. PMID 18216771. http://xa.yimg.com/kq/groups/15186538/1872049761/name/panthini.pdf.
- ↑ Michael Podeschwa, Oliver Plettenburg, Jochen vom Brocke, Oliver Block, Stephan Adelt, Hans-Josef Altenbach (2003): "Stereoselective synthesis of myo-, neo-, L-chiro, D-chiro, allo-, scyllo-, and epi-inositol systems via conduritols prepared from p-benzoquinone". European Journal of Organic Chemistry, volume 2003, issue 10, pages 1958-1972. doi:10.1002/ejoc.200200572
- ↑ Irina Monnard, Thierry Bénet, Rosemarie Jenni, Sean Austin, Irma Silva-Zolezzi, Jean-Philippe Godin (2020): "Plasma and urinary inositol isomer profiles measured by UHPLC-MS/MS reveal differences in scyllo-inositol levels between non-pregnant and pregnant women". Analytical and Bioanalytical Chemistry, volume 412, pages 7871–7880. doi:10.1007/s00216-020-02919-8
 |
