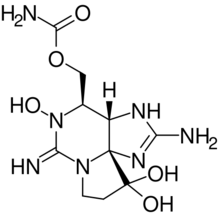
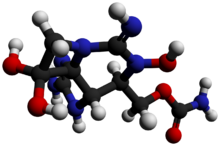
Chemistry:Neosaxitoxin
| |
| |
| Names | |
|---|---|
| IUPAC name | |
| Other names
1H,10H-Pyrrolo(1,2-c)purine-10,10-diol, 2-amino-4-(((aminocarbonyl)oxy)methyl)-3a,4,5,6,8,9-hexahydro-5-hydroxy-6-imino-, (3aS,4R,10aS)-; 1H,10H-Pyrrolo(1,2-c)purine-10,10-diol, 2-amino-4-((aminocarbonyl)oxy)methyl-3a,4,5,6,8,9-hexahydro-5-hydroxy-6-imino-,(3aS,4R,10aS)-; 1H,10H-Pyrrolo(1,2-c)purine-10,10-diol, 2-amino-4-((aminocarbonyl)oxy)methyl-3a,4,5,6,8,9-hexahydro-5-hydroxy-6-imino-,(3aS-(3aalpha,4alpha,10aR*))-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H17N7O5 | |
| Molar mass | 315.286 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H300 | |
| P264, P270, P301+310, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Neosaxitoxin (NSTX) is included, as other saxitoxin-analogs, in a broad group of natural neurotoxic alkaloids, commonly known as the paralytic shellfish toxins (PSTs). The parent compound of PSTs, saxitoxin (STX), is a tricyclic perhydropurine alkaloid, which can be substituted at various positions, leading to more than 30 naturally occurring STX analogues. All of them are related imidazoline guanidinium derivatives.[3]
Sources
NSTX, and other PSTs, are produced by several species of marine dinoflagellates (eukaryotes) and freshwater cyanobacteria, blue-green algae (prokaryotes), which can form extensive blooms around the world.[4] Under special conditions, during harmful algal blooms (HAB) or red tide, all these toxins may build up in filter-feeding shellfish, such as mussels, clams and oysters, and can produce an outbreak of Paralytic Shellfish Poisoning (PSP).[5]
Saxitoxin analogues associated to PSP can be divided into three categories:[6]
- Carbamate compounds, including saxitoxin, neosaxitoxin and gonyautoxins 1–4.
- N-sulfocarbamoyl compounds, including C and B toxins.
- Decarbamoyl compounds with respect to the presence or absence of 1-N-hydroxyl, 11-hydroxysulfate, and 21-N-sulfocarbamoyl substitutions as well as epimerization at the C-11 position.
Structure and properties
NSTX is quite similar to saxitoxin, like all the neurotoxins associated to PSP, the only difference is that NSTX shows one hydroxyl group bonded to nitrogen "1", where saxitoxyn contains one hydrogen.[7]
This purine is highly hydrophilic[8] and thermostable, it is not destroyed by cooking.[9] Moreover, is very stable in usual storage, specially in acidic condition.[10]
Mechanism of action
NSTX blocks the extracellular portion,[11] the outer vestibule,[12] of some voltage gated sodium channels in a very powerful and reversible manner, without affection of other ion channels.
"Voltage-gated", also called "voltage-sensitive" and "voltage-dependent" sodium channel also known as "VGSCs" or "Nav channel" are crucial elements of normal physiology in a variety of animals, including flies, leeches, squid and jellyfish, as well as mammalian and non-mammalian vertebrates. This large integral membrane protein plays an essential role in the initiation and propagation of action potentials in neurons, myocytes and other excitable cells.[13]
Nav channels form the basis for electrical excitability in animals. Nav channels evolved from Ca2+ channels and were present in the common ancestor of choanoflagellates and animals, although this channel was likely permeable to both Na+ and Ca2+. Thus, like many other neuronal channels and receptors, Nav channels predated neurons. Invertebrates possess two Nav channels (Nav1 and Nav2), whereas vertebrate Nav channels are of the Nav1 family.[14]
Sodium-channel proteins in the mammalian brain are composed of an association that include one alpha subunit and one or more auxiliary beta subunits. Nine types of alpha subunits have been described (Nav1.1 to Nav1.9), and a tenth related isoform (Nax) may also play some role as a Nav channel. Based in this information, ten Nav classes can be described: Nav1.1 to Nav1.9, and Nax.[15]
Former five,[16] but more recently, six[17] neurotoxin receptor sites have been recognized between the seven receptor site[18] located in the vertebrate sodium channel receptor alpha subunit:
- Site 1 binds the sodium channel blockers tetrodotoxin and saxitoxin.
- Site 2 binds lipid-soluble sodium channel activators such as veratridine.
- Site 3 binds alpha-scorpion and sea anemone toxins, which slow sodium channel inactivation.
- Site 4 binds beta-scorpion toxins, which affect sodium channel activation.
- Site 5 binds the polyether ladder brevetoxins and ciguatoxin.
- Site 6 binds delta-conotoxin.
- Local anesthetic receptor site binds local anesthetics, antiarrhythmic drugs and antiepileptic drugs
NSTX and other site 1 blockers have high affinity (very low dissociation constant) and high specificity for Nav channels. The action of NSTX produces minimal effect on cardiac Nav, where it exhibits about 20–60 fold lesser affinity than in Nav channels from rat skeletal muscle and rat brain.[19] Most data emphasize the role of "STX resistant" Nav channel 1.5 in human heart.[20][21]
Toxins such as neosaxitoxin and tetrodotoxin have less affinity for most cardiac Nav channels than for most Nav channels in nerve tissue. Moreover, NSTX is so active on nerve Nav channel than is roughly a million-fold more potent than lidocaine.[22]
Effects on humans
This mechanism of action can produce two well known kinds of effects in humans:
Toxic effect, associated to plasmatic levels of NSTX
It can be approximately described using one of the classical model of neurotoxic disease, known from ancient times as red tide, the most harmful algal bloom (HAB). This well known clinical model is the "paralytic shellfish poisoning".[23]
Of course, there are great differences between different algal blooms,[24][25][26][27] because of the mix of species included in each HAB, usually related to environmental conditions;[28] because of the levels and quality of PSTs produced in each HAB, that may be modulated by concurrent microorganism;[29][30][31][32] and, last but not least, because of the specific properties of each kind of PST, for example:
- Brevetoxins are lipid-soluble (hydrophobic) polyether marine toxins; their predominant effect is excitatory (blocked by tetrodotoxin), mediated by the enhancement of cellular Na+ influx; and bind to site 5 on Nav (like ciguatoxin).[33]
- Tetrodotoxin (TTX) toxicity is associated with marked and surprising cardiovascular effects (i.e.: hypotension and bradycardia).[34] Those effects are unexpected because of notorious TTX-resistance observed in vertebrate cardiac Nav channel. Moreover, this characteristic of the mammalian cardiac Nav channel is attributed to the cardiac predominance of the TTX-resistant Nav channel isoform (Nav1.5).[35] On the contrary, as presumed on physiologic basis, NSTX produces just mild and transient cardiovascular abnormalities during experimental intoxication (there are no data on pure NSTX clinical toxicity).
- STX has two positive charges, in contrast to TTX's single charge and GTX2/3, a naturally occurring STX congener with a net +1 charge. In view of their rather different structures, it is not surprising that STX and TTX bind in a different fashion to VGSCs. In fact, when Phe 385 near the selectivity filter of Nav1.2 is mutated to Cys, the channel's affinity for TTX is reduced 3,000-fold, whereas that for STX is reduced (only) 340-fold.[36]
- There are very limited data on the relative potency of different PSTs, and developing alternative methods to animal bioassays for marine-toxin detection is an urgent need.[37]
In spite of its heterogeneous and poorly understood epidemiology, the clinical picture of PSP could be useful to anticipate clinical effects of systemic NSTX.
- In the most frequent and benign situation, the patient suffers just mild, short-lived paresthesias of the mouth or extremities.
- In moderate cases perioral tingling progressing to numbness spreading to face and neck can be observed.[38]
- In severe cases, patient can suffer apnea secondary to motor block, requiring mechanical ventilation.[39]
Usually, the victims of mild and severe acute intoxications eliminate the toxin in urine during the first 24 hours after ingestion, and improve to full recovery in the first day of intrahospital care (when vital support is provided in a timely manner).[40]
When outbreaks of PSP occur in remote locations, where medical assistance is limited, reported lethality is under 10% in adults, but can reach 50% in children younger than six years old. This difference could be secondary to dissimilar doses and composition of involved mixes of PSTs; delay in medical support; or some kind of susceptibility of children.[41] More recent information suggest that lethality could be around 1% of symptomatic patients,[42] including cases where air transportation was required from remote locations of Alaska.[43]
Electrophysiologic observations demonstrated sub clinical abnormalities lasting for some days[44] or weeks[45] after clinical recovery .
Some evidence suggest the presence of metabolic pathways for the sequential oxidation and glucuronidation of PST in vitro, both being the initial detoxication reactions for the excretion of these toxins in humans.[46]
Forensic analysis of fatalities after severe cases, conclude that PSP toxins are metabolically transformed by humans and that they are removed from the body by excretion in the urine and feces like any other xenobiotic compound.[47]
Considering the heterogeneous nature of toxins mixes contained in contaminated bivalve molluscs, the safe limit of toxin content in shellfish adequate for human ingestion is expressed in "saxitoxin equivalents". According to the Food and Agriculture Organization of the United Nations (FAO) and European Parliament, this limit is 80 microgram of saxitoxin equivalent per 100 gram of mussel meat (each mussel weights around 23 g).[48][49] The U.S. Food and Drug Administration extends the same definition to "fish" quality, but the term "fish" refers to fresh or saltwater fin fish, crustaceans, other forms of aquatic animal life other than birds or mammals, and all mollusks; and incorporate the use of "ppm" as another measure for saxitoxin equivalent concentration in mentioned foods.[50]
Paradoxically, the chronic and/or repeated exposure to marine seafood toxins, which is a much more realistic phenomenon, has not been fully examined.[51][52] One study in rats exposed to chronic (12 weeks) NSTX administration demonstrated some reduction in water and food intake, and a mild degree of transient cholestasis, probably associated to fasting, without other abnormalities.[53]
Anesthetic effect, produced by local infiltration of NSTX
This action has been demonstrated in animals[54] and humans.[55][56][57][58][59]
The medical use of the NSTX anesthetic effect is supported by three reasons:
- NSTX anesthetic duration:
- Any current available local anesthetic hardly produces clinical effects 12 hours after a single injection.[60] Then, in cases of severe or prolonged pain, some patients need repeated injections, catheters, pumps and opioids[61][62] to feel comfortable, with different kinds of side effects, costs and risks.[63]
- On the other hand, NSTX local infiltration produces long lasting anesthesia, well over all the current available local anesthetics. Some investigations demonstrated anesthetic effect lasting over one week after single injection in rodents, using extended release formulation, without histologic or functional sequelae.[64]
- Additionally, two human reports demonstrated strong potentiation between NSTX anesthetic effect, bupivacaine and epinephrine.[65][66]
- NSTX local safety:
- All available local anesthetic are associated with local damage in different models.[67][68][69][70][71] This undesired effect could be enhanced by sustained release formulations.[72]
- On the contrary, several investigations show local safety of saxitoxin-related neurotoxins, including very sensitive models, and there is no reason to presume otherwise for NSTX.[73][74][75][76]
- NSTX systemic safety:
- In spite of advances of ultrasound guided injections, acute systemic local anesthetic toxicity is still an unsolved clinical problem, and can produce devastating consequences, related to the neurologic and cardiovascular effects of all available local anesthetics.[77][78]
- Otherwise, clinical experience and animal models shows the relative safety of accidental and experimental NSTX intoxication (when appropriate support therapy is provided in a timely manner).[79]
- Recent investigation in sheep shows a safe limit, due to motor block, over 1 µg/kg for intravenous injection of NSTX, with full recovery after a brief course of mechanical ventilation.[80]
- Regarding systemic safety, saxitoxins diffuse through the blood–brain barrier,[81] but, because of Nav channel specificity, acute toxicity is associated to a very low risk of seizures. This establishes an important difference with current local anesthetic toxicity.[82]
- As could be predicted from its ion channel selectivity,[83] NSTX intoxication clinical picture is almost devoid of arrhythmias, establishing another difference with available local anesthetic's numerous cardiac effects.[84]
- And last but not least, some degree of improving in therapeutic index of NSTX can be observed when is mixed with bupivacaine and/or epinephrine.[85]
In conclusion, NSTX is a well defined molecule with a long-lasting and sometimes dangerous relationship with human subjects. Recent investigations suggest a clinical application as a new local anesthetic that sounds "too good to be true", but more investigation is required.[86]
See also
References
- ↑ United States National Library of Medicine (NLM). ChemoIDplus Advanced. Registry number: 64296-20-4 (accessed: May 12, 2012) [1]
- ↑ National Center for Biotechnology Information (NCBI). PubChem Compound (accessed: May 12, 2012) [2]
- ↑ "Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5". BMC Biochemistry 10: 8. March 2009. doi:10.1186/1471-2091-10-8. PMID 19331657.
- ↑ "Neurotoxic Alkaloids: Saxitoxin and Its Analogs". Marine Drugs 8 (7): 2185–2211. July 2010. doi:10.3390/md8072185. PMID 20714432.
- ↑ Centers for Disease Control and Prevention (CDC). CDC's Laboratory Response to Toxins (accessed: May 8, 2012) [3]
- ↑ Wang DZ (March 2008). "Neurotoxins from Marine Dinoflagellates: A Brief Review". Marine Drugs 6 (3): 349–71. doi:10.3390/md6020349. PMID 18728731.
- ↑ "Interactions of neosaxitoxin with the sodium channel of the frog skeletal muscle fiber". The Journal of General Physiology 97 (3): 561–78. March 1991. doi:10.1085/jgp.97.3.561. PMID 1645395.
- ↑ Etheridge SM (August 2010). "Paralytic shellfish poisoning: seafood safety and human health perspectives". Toxicon 56 (2): 108–22. doi:10.1016/j.toxicon.2009.12.013. PMID 20035780. https://zenodo.org/record/1259399.
- ↑ "Effect of cooking on the concentration of toxins associated with paralytic shellfish poison in lobster hepatopancreas". Toxicon 32 (1): 57–64. January 1994. doi:10.1016/0041-0101(94)90021-3. PMID 9237337.
- ↑ "Comparative study of the stability of saxitoxin and neosaxitoxin in acidic solutions and lyophilized samples". Toxicon 32 (12): 1593–8. December 1994. doi:10.1016/0041-0101(94)90318-2. PMID 7725328.
- ↑ "Blockers of Voltage-Gated Sodium Channels for the Treatment of Central Nervous System Diseases". Recent Patents on CNS Drug Discovery 2 (1): 57–78. January 2007. doi:10.2174/157488907779561754. PMID 18221218.
- ↑ "Specific neosaxitoxin interactions with the Na+ channel outer vestibule determined by mutant cycle analysis". Biophysical Journal 80 (2): 698–706. February 2001. doi:10.1016/S0006-3495(01)76049-3. PMID 11159437. Bibcode: 2001BpJ....80..698P.
- ↑ Catterall WA. (April 2012). "Voltage-Gated Sodium Channels at 60: Structure, Function, and Pathophysiology". The Journal of Physiology 590 (Pt 11): 2577–89. doi:10.1113/jphysiol.2011.224204. PMID 22473783.
- ↑ Zakon H. (June 2012). "Adaptive evolution of voltage-gated sodium channels: the first 800 million years". Proceedings of the National Academy of Sciences of the United States of America 109 (Suppl 1): 10619–25. doi:10.1073/pnas.1201884109. PMID 22723361. Bibcode: 2012PNAS..10910619Z.
- ↑ "Overview of the voltage-gated sodium channel family". Genome Biology 4 (3): 207. July 2003. doi:10.1186/gb-2003-4-3-207. PMID 12620097.
- ↑ "A new neurotoxin receptor site on sodium channels is identified by a conotoxin that affects sodium channel inactivation in molluscs and acts as an antagonist in rat brain". Recent Patents on CNS Drug Discovery 269 (4): 2574–80. January 1994. PMID 8300586. http://www.jbc.org/content/269/4/2574.full.pdf. Retrieved May 9, 2012.
- ↑ "Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor". PNAS 108 (37): 15426–31. September 2011. doi:10.1073/pnas.1112320108. PMID 21876146. Bibcode: 2011PNAS..10815426W.
- ↑ "International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels". Pharmacological Reviews 57 (4): 397–409. December 2005. doi:10.1124/pr.57.4.4. PMID 16382098.
- ↑ "Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle". Biochemistry 26 (24): 7546–56. December 1987. doi:10.1021/bi00398a003. PMID 2447944.
- ↑ "Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway". The Journal of Cell Biology 180 (1): 173–86. January 2008. doi:10.1083/jcb.200710107. PMID 18180363.
- ↑ Abriel H (May 7, 2012). "Cardiac Sodium Channel Nav1.5 Mechanosensitivity is Inhibited by Ranolazine". Circulation 125 (22): 2681–3. doi:10.1161/CIRCULATIONAHA.112.110908. PMID 22565937.
- ↑ Butterworth JF 4th (March–April 2011). "Will conventional local anesthetics soon be replaced by neurotoxins?". Regional Anesthesia and Pain Medicine 36 (2): 101–2. doi:10.1097/AAP.0b013e31820db23e. PMID 21326065.
- ↑ FAO FOOD AND NUTRITION PAPER 80. Food and Agriculture Organization of the United Nations. Chapter 2. Paralytic Shellfish Poisoning (PSP). Rome, 2004(accessed: May 6, 2012)[4][yes|permanent dead link|dead link}}]
- ↑ "Intoxicaciones por toxina paralizante de molusco en Oaxaca" (in es). Salud Pública de México 33 (3): 240–7. May–June 1991. https://www.redalyc.org/pdf/106/10633306.pdf. Retrieved May 10, 2012.
- ↑ Anderson DM (August 1994). "Red tides". Scientific American 271 (2): 62–8. doi:10.1038/scientificamerican0894-62. PMID 8066432. Bibcode: 1994SciAm.271b..62A. (accessed: May 9, 2012) [5]
- ↑ "3. Cyanobacterial Toxins". Toxic Cyanobacteria in Water: A guide to their public health consequences, monitoring and management. World Health Organization. 1999. ISBN 0-419-23930-8. https://www.who.int/water_sanitation_health/resourcesquality/toxicyanbact/en/index.html. Retrieved 10 May 2012. [6]
- ↑ "The Global, Complex Phenomena of Harmful Algal Blooms". Oceanography 18 (2): 136–47. June 2005. doi:10.5670/oceanog.2005.49.(accessed: May 11, 2012) [7]
- ↑ "Analysis of change of red tide species in Yodo River estuary by the numerical ecosystem model". Marine Pollution Bulletin 57 (1–5): 103–7. 2008. doi:10.1016/j.marpolbul.2008.04.015. PMID 18513758. Bibcode: 2008MarPB..57..103H. http://www.lib.kobe-u.ac.jp/repository/90000799.pdf.
- ↑ "Evidence for production of paralytic shellfish toxins by bacteria associated with Alexandrium spp. (Dinophyta) in culture". Applied and Environmental Microbiology 63 (1): 239–45. January 1997. doi:10.1128/AEM.63.1.239-245.1997. PMID 9065273. Bibcode: 1997ApEnM..63..239G.
- ↑ "Evidence of a new toxin in the red-tide dinoflagellate Prorocentrum minimum". Journal of Plankton Research 19 (8): 1111–24. August 1997. doi:10.1093/plankt/19.8.1111.
- ↑ "Microbial modulation in the biomass and toxin production of a red-tide causing alga". Marine Pollution Bulletin 51 (8–12): 1018–25. 2005. doi:10.1016/j.marpolbul.2005.02.039. PMID 16291201. Bibcode: 2005MarPB..51.1018Z.
- ↑ "Grazing of heterotrophic dinoflagellate Noctiluca scintillans (Mcartney) Kofoid on Gymnodinium catenatum Graham". Revista Latinoamericana de Microbiología 47 (1–2): 6–10. January–June 2005. PMID 17061541.
- ↑ Baden DG (May 1989). "Brevetoxins: unique polyether dinoflagellate toxins". The FASEB Journal 3 (7): 1807–17. doi:10.1096/fasebj.3.7.2565840. PMID 2565840.
- ↑ "Tetrodotoxin poisoning". The American Journal of Emergency Medicine 21 (1): 51–4. January 2003. doi:10.1053/ajem.2003.50008. PMID 12563582.
- ↑ "Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current". Marine Drugs 9 (11): 2409–22. 2011. doi:10.3390/md9112409. PMID 22163193.
- ↑ "Cooccupancy of the outer vestibule of voltage-gated sodium channels by micro-conotoxin KIIIA and saxitoxin or tetrodotoxin". Journal of Neurophysiology 104 (1): 88–97. July 2010. doi:10.1152/jn.00145.2010. PMID 20410356.
- ↑ "Determination of toxicity equivalent factors for paralytic shellfish toxins by electrophysiological measurements in cultured neurons". Chemical Research in Toxicology 24 (7): 1153–7. July 2011. doi:10.1021/tx200173d. PMID 21619049.
- ↑ Woods Hole Oceanographic Institution. Paralytic Shellfish Poisoning. Fleming LE. Last updated: May 7, 2008 (accessed: May 8, 2012)[8]
- ↑ "Respiratory distress after consumption of sea snails". Hong Kong Journal of Emergency Medicine 9 (3): 159–61. April 2002. doi:10.1177/102490790200900308. http://www.hkcem.com/html/publications/Journal/2002-3/159-161.pdf. Retrieved May 6, 2012.
- ↑ Centers for Disease Control and Prevention (CDC) (December 2011). "Paralytic Shellfish Poisoning Southeast Alaska, May–June 2011". Morbidity and Mortality Weekly Report 60 (45): 1554–56. PMID 22089968.(accessed: May 8, 2012)[9]
- ↑ "Lethal paralytic shellfish poisoning in Guatemala". American Journal of Tropical Medicine and Hygiene 42 (3): 267–71. March 1990. doi:10.4269/ajtmh.1990.42.267. PMID 2316796.
- ↑ "Creatine kinase MB elevation in paralytic shellfish poisoning". Chest 99 (4): 1032–3. April 1991. doi:10.1378/chest.99.4.1032. PMID 2009759.
- ↑ "Paralytic shellfish poisoning in Alaska: a 20-year retrospective analysis". American Journal of Epidemiology 141 (8): 766–70. April 1995. doi:10.1093/oxfordjournals.aje.a117499. PMID 7709919.
- ↑ "Paralytic shellfish poisoning. A case report and serial electrophysiologic observations". Neurology 40 (8): 1310–2. August 1990. doi:10.1212/wnl.40.8.1310. PMID 2381544.
- ↑ "Paralytic Paralytic shellfish poisoning: clinical and electrophysiological observations". Journal of Neurology 245 (8): 551–4. August 1998. doi:10.1007/s004150050241. PMID 9747920.
- ↑ "Paralytic Route of metabolization and detoxication of paralyticshellfishtoxins in humans". Toxicon 55 (1): 135–44. January 2010. doi:10.1016/j.toxicon.2009.07.018. PMID 19632259.
- ↑ "Paralytic shellfish poisoning: post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords". Toxicon 43 (2): 149–58. February 2004. doi:10.1016/j.toxicon.2003.11.018. PMID 15019474.
- ↑ FAO Food and Nutrition Paper 80. Food and Agriculture Organization of the United Nations. Chapter 8. Risk Assessment. Rome, 2004. (accessed: May 6, 2012) [10][yes|permanent dead link|dead link}}]
- ↑ The European Parliament and the Council of the European Union (April 2004). "Regulation (EC) N° 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin". Off J Eur Comm 139: 61. (accessed: May 6, 2012)[11]
- ↑ U.S. Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guidance, Fourth Edition, November 2011. Chapter 6: Natural Toxins (p. 99–112). (accessed: May 6, 2012) [12] [13]
- ↑ Woods Hole Oceanographic Institution. Marine Biotoxins and Harmful Algae: A National Plan. Chapter II. THE TOXINS. Risk Assessment. (accessed: May 8, 2012)[14]
- ↑ "Risk of human exposure to paralytic toxins of algal origin". Environmental Toxicology and Pharmacology 19 (3): 401–6. May 2005. doi:10.1016/j.etap.2004.12.002. PMID 21783504.
- ↑ "Chronic Toxicity Study of Neosaxitoxin in Rats". Marine Drugs 12 (9): 5055–71. September 2014. doi:10.3390/md12095055. PMID 25257789.
- ↑ "The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo". Regional Anesthesia and Pain Medicine 25 (1): 52–9. Jan–Feb 2000. doi:10.1097/00115550-200001000-00010. PMID 10660241.
- ↑ "Intrasphincteric neosaxitoxin injection: evidence of lower esophageal sphincter relaxation in achalasia". American Journal of Gastroenterology 101 (11): 2667–8. November 2006. doi:10.1111/j.1572-0241.2006.00809_6.x. PMID 17090291.
- ↑ "Neosaxitoxin as a local anesthetic: preliminary observations from a first human trial". Anesthesiology 106 (2): 339–45. February 2007. doi:10.1097/00000542-200702000-00023. PMID 17264729.
- ↑ "Comparison of neosaxitoxin versus bupivacaine via port infiltration for postoperative analgesia following laparoscopic cholecystectomy: a randomized, double-blind trial". Regional Anesthesia and Pain Medicine 36 (2): 103–9. March–April 2011. doi:10.1097/aap.0b013e3182030662. PMID 21425506.
- ↑ "First evidence of neosaxitoxin as a long-acting pain blocker in bladder pain syndrome". Int Urogynecol J 26 (6): 853–8. 2015. doi:10.1007/s00192-014-2608-2. PMID 25571865.
- ↑ Coppens SJR, Zawodny Z, Dewinter G, Neyrinck A, Balocco AL, Rex S. In search of the Holy Grail: Poisons and extended release local anesthetics. Best Pract Res Clin Anaesthesiol. 2019 Mar;33(1):3-21. doi:10.1016/j.bpa.2019.03.002 PMID 31272651
- ↑ "Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine". Acta Biomedica 79 (2): 92–105. August 2008. PMID 18788503. (accessed: May 10, 2012) [15]
- ↑ "Postoperative intravenous morphine titration". British Journal of Anaesthesia 108 (2): 193–201. February 2012. doi:10.1093/bja/aer458. PMID 22250276.
- ↑ "Focused review: ropivacaine versus bupivacaine for epidural labor analgesia". Anesthesia & Analgesia 111 (2): 482–7. August 2012. doi:10.1213/ANE.0b013e3181e3a08e. PMID 20529986.
- ↑ "Treatment of acute postoperative pain". Lancet 377 (9784): 2215–25. June 2011. doi:10.1016/S0140-6736(11)60245-6. PMID 21704871.
- ↑ "Prolonged duration local anesthesia with minimal toxicity". Proceedings of the National Academy of Sciences of the United States of America 106 (17): 7125–30. April 2009. doi:10.1073/pnas.0900598106. PMID 19365067. Bibcode: 2009PNAS..106.7125E.
- ↑ "Potentiation of local anesthetic activity of neosaxitoxin with bupivacaine or epinephrine: development of a long-acting pain blocker". Neurotoxicity Research 16 (4): 408–15. November 2009. doi:10.1007/s12640-009-9092-3. PMID 19636660.
- ↑ "A Phase 1, Dose-escalation, Double-blind, Block-randomized, Controlled Trial of Safety and Efficacy of Neosaxitoxin Alone and in Combination with 0.2% Bupivacaine, with and without Epinephrine, for Cutaneous Anesthesia". Anesthesiology 123 (4): 873–85. October 2015. doi:10.1097/ALN.0000000000000831. PMID 26275090.
- ↑ "Review Articles: Local Anesthetic Myotoxicity". Regional Anesthesia and Pain Medicine 29 (4): 333–40. July–August 2004. doi:10.1016/j.rapm.2004.02.008. PMID 15305253.
- ↑ "Cytotoxicity of lidocaine or bupivacaine on corneal endothelial cells in a rabbit model". Cornea 25 (5): 590–6. June 2006. doi:10.1097/01.ico.0000220775.93852.02. PMID 16783149.
- ↑ "Cytotoxicity of local anesthetics in human neuronal cells". Anesthesia & Analgesia 108 (3): 997–1007. March 2009. doi:10.1213/ane.0b013e31819385e1. PMID 19224816.
- ↑ "Age-dependent bupivacaine-induced muscle toxicity during continuous peripheral nerve block in rats". Anesthesiology 111 (5): 1120–7. November 2009. doi:10.1097/ALN.0b013e3181bbc949. PMID 19809284.
- ↑ "Is chemical incompatibility responsible for chondrocyte death induced by local anesthetics?". The American Journal of Sports Medicine 38 (3): 520–6. March 2010. doi:10.1177/0363546509349799. PMID 20194957.
- ↑ "Local Myotoxicity from Sustained Release of Bupivacaine from Microparticles". Anesthesiology 108 (5): 921–8. May 2008. doi:10.1097/ALN.0b013e31816c8a48. PMID 18431129.
- ↑ "Comparative cytotoxicity of aflatoxin B1 and saxitoxin in cell cultures". Molecular Toxicology 1 (2–3): 209–16. April–September 1987. PMID 3130568.
- ↑ "Tetrodotoxin: anesthetic activity in the de-epithelialized cornea". Graefe's Archive for Clinical and Experimental Ophthalmology 236 (10): 790–4. October 1998. doi:10.1007/s004170050160. PMID 9801896.
- ↑ "Saxitoxin: an anesthetic of the deepithelialized rabbit cornea". Cornea 20 (6): 639–42. August 2001. doi:10.1097/00003226-200108000-00016. PMID 11473167.
- ↑ "Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity". Muscle Nerve 34 (6): 747–53. December 2006. doi:10.1002/mus.20618. PMID 16897761.
- ↑ "Local anesthetic systemic toxicity". Canadian Journal of Anesthesia 57 (4): 368–80. April 2010. doi:10.1007/s12630-010-9275-7. PMID 20151342.
- ↑ "ASRA practice advisory on local anesthetic systemic toxicity.". Regional Anesthesia and Pain Medicine 35 (2): 152–61. March–April 2010. doi:10.1097/AAP.0b013e3181d22fcd. PMID 20216033.
- ↑ "Paralytic shellfish poisoning. A report of 17 cases in Cape Town". South African Medical Journal 55 (25): 1017–23. June 1979. PMID 573505. (accessed: May 8, 2012)[16]
- ↑ "Respiratory, neuromuscular, and cardiovascular effects of neosaxitoxin in isoflurane-anesthetized sheep". Regional Anesthesia and Pain Medicine 37 (2): 152–8. March 2012. doi:10.1097/AAP.0b013e3182424566. PMID 22330260.
- ↑ "Human Health Risk Assessment Related to Cyanotoxins Exposure". Critical Reviews in Toxicology 38 (2): 97–125. February 2008. doi:10.1080/10408440701749454. PMID 18259982.
- ↑ Guay J (December 2009). "Adverse events associated with intravenous regional anesthesia (Bier block): a systematic review of complications". Journal of Clinical Anesthesia 21 (8): 585–94. doi:10.1016/j.jclinane.2009.01.015. PMID 20122591.
- ↑ "Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle". Biochemistry 26 (24): 7546–56. December 1987. doi:10.1021/bi00398a003. PMID 2447944.
- ↑ "Sodium channel molecular conformations and antiarrhythmic drug affinity". Trends in Cardiovascular Medicine 20 (1): 16–21. January 2010. doi:10.1016/j.tcm.2010.03.002. PMID 20685573.
- ↑ "Neosaxitoxin in Rat Sciatic Block: Improved Therapeutic Index Using Combinations with Bupivacaine, with and without Epinephrine.". Anesthesiology 123 (4): 886–98. October 2015. doi:10.1097/ALN.0000000000000832. PMID 26280473.
- ↑ "Novelty without toxicity: a quest for a safer local anesthetic". Canadian Journal of Anesthesia 58 (1): 8–13. January 2011. doi:10.1007/s12630-010-9409-y. PMID 21042902.
 |
