Chemistry:Nitrilimine
Nitrilimines or nitrile amides are a class of organic compounds sharing a common functional group with the general structure R-CN-NR corresponding to the conjugate base of an amine bonded to the N-terminus of a nitrile. The dominant structure for the parent compound nitrilimine is that of the propargyl-like 1 in scheme 1 with a C-N triple bond and with a formal positive charge on nitrogen and two lone pairs and a formal negative charge on the terminal nitrogen. Other structures such as hypervalent 2, allene-like 3, allylic 4 and carbene 5 are of lesser relevance.
| [math]\displaystyle{ \ce{H-\!{\equiv}}{\color{Blue}\ce{N+}}\!{-}\!{\color{Red}\ce{N-}}\!{-}\!{\color{Red}\ce H} }[/math] |
[math]\ce{ H-\!{\equiv}N=N-H }[/math] |
[math]\displaystyle{ \ce H\!{-}\!{\color{Red} \ce{C-}}\!{=}{\color{Blue}\ce{N+}}\!\ce{=N-H} }[/math] |
[math]\displaystyle{ \ce H\!{-}\!{\color{Blue} \ce{C+}}\!\ce{=N}\!{-}\!{\color{Red}\ce{N-}}\!\ce{-H} }[/math] |
[math]\ce{ H-C{:}\!-N=N-H }[/math] |
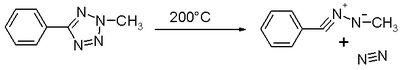
Nitrilimines were first observed in the thermal decomposition of 2-tetrazoles releasing nitrogen:[1]
-
()
Nitrilimines are linear 1,3-dipoles represented by structures 1 and 3. A major use is in heterocyclic synthesis. E.g. with alkynes they generate pyrazoles in a 1,3-dipolar cycloaddition. Due to their high energy, they are usually generated in situ as a reactive intermediate.
References
- ↑ Huisgen, Rolf; Seidel, Michael; Sauer, Juergen; McFarland, James; Wallbillich, Guenter (June 1959). "Communications: The Formation of Nitrile Imines in the Thermal Breakdown of 2,5-Disubstituted Tetrazoles". The Journal of Organic Chemistry 24 (6): 892–893. doi:10.1021/jo01088a034.
 |