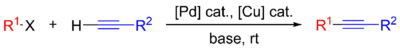Chemistry:Alkyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond.[1] The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula C
nH
2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C
2H
2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.[2]
Structure and bonding
In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm).[3]

Illustrative alkynes: a, acetylene, b, two depictions of propyne, c, 1-butyne, d, 2-butyne, e, the naturally occurring 1-phenylhepta-1,3,5-triyne, and f, the strained cycloheptyne. Triple bonds are highlighted blue.
The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes 369 kJ/mol, the first pi bond contributes 268 kJ/mol. The second pi bond 202 kJ/mol. Bonding is usually discussed in the context of molecular orbital theory, which recognizes triple bond arising from the overlap of s and p orbitals. In terms of valence bond theory, the carbon atoms in an alkyne bond are sp hybridized which means they each have two unhybridized p orbitals and two sp hybrid orbitals. Overlap of an sp orbital from each atom forms one sp–sp sigma bond. Each p orbital on one atom overlaps one on the other atom, forming two pi bonds, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom. For example, to hydrogen atoms in the parent acetylene. The two sp orbitals project on opposite sides of the carbon atom.
Terminal and internal alkynes
Internal alkynes feature carbon substituents on each acetylenic carbon. Symmetrical examples include diphenylacetylene and 3-hexyne. They may also be asymmetrical, such as in 2-pentyne.
Terminal alkynes have the formula RC≡CH, where at least one end of the alkyne is a hydrogen atom. An example is methylacetylene (propyne using IUPAC nomenclature). They are often prepared by alkylation of monosodium acetylide.[4] Terminal alkynes, like acetylene itself, are mildly acidic, with pKa values of around 25. They are far more acidic than alkenes and alkanes, which have pKa values of around 40 and 50, respectively. The acidic hydrogen on terminal alkynes can be replaced by a variety of groups resulting in halo-, silyl-, and alkoxoalkynes. The carbanions generated by deprotonation of terminal alkynes are called acetylides.[5] Internal alkynes are also considerably more acidic than alkenes and alkanes, though not nearly as acidic as terminal alkynes. The C–H bonds at the α position of alkynes (propargylic C–H bonds) can also be deprotonated using strong bases, with an estimated pKa of 35. This acidity can be used to isomerize internal alkynes to terminal alkynes using the alkyne zipper reaction.
Naming alkynes
In systematic chemical nomenclature, alkynes are named with the Greek prefix system without any additional letters. Examples include ethyne or octyne. In parent chains with four or more carbons, it is necessary to say where the triple bond is located. For octyne, one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. The lowest number possible is given to the triple bond. When no superior functional groups are present, the parent chain must include the triple bond even if it is not the longest possible carbon chain in the molecule. Ethyne is commonly called by its trivial name acetylene.
In chemistry, the suffix -yne is used to denote the presence of a triple bond. In organic chemistry, the suffix often follows IUPAC nomenclature. However, inorganic compounds featuring unsaturation in the form of triple bonds may be denoted by substitutive nomenclature with the same methods used with alkynes (i.e. the name of the corresponding saturated compound is modified by replacing the "-ane" ending with "-yne"). "-diyne" is used when there are two triple bonds, and so on. In case of multiple triple bonds, the position of unsaturation is indicated by a numerical locant immediately preceding the "-yne" suffix, or 'locants'. Locants are chosen so that the numbers are low as possible. "-yne" is also used as a suffix to name substituent groups that are triply bound to the parent compound.
Sometimes a number between hyphens is inserted before it to state which atoms the triple bond is between. This suffix arose as a collapsed form of the end of the word "acetylene". The final "-e" disappears if it is followed by another suffix that starts with a vowel.[6]
Structural isomerism
Alkynes having four or more carbon atoms can form different structural isomers by having the triple bond in different positions or having some of the carbon atoms be substituents rather than part of the parent chain. Other non-alkyne structural isomers are also possible.
- C
2H
2: acetylene only - C
3H
4: propyne only - C
4H
6: 2 isomers: 1-butyne, and 2-butyne - C
5H
8: 3 isomers: 1-pentyne, 2-pentyne, and 3-methyl-1-butyne - C
6H
10: 7 isomers: 1-hexyne, 2-hexyne, 3-hexyne, 4-methyl-1-pentyne, 4-methyl-2-pentyne, 3-methyl-1-pentyne, 3,3-dimethyl-1-butyne
Synthesis
From calcium carbide
Classically, acetylene was prepared by hydrolysis (protonation) of calcium carbide (Ca2+[:C≡C:]2–):
which was in turn synthesized by combining quicklime and coke in an electric arc furnace at 2200 °C:
This was an industrially important process which provided access to hydrocarbons from coal resources for countries like Germany and China. However, the energy-intensive nature of this process is a major disadvantage and its share of the world's production of acetylene has steadily decreased relative to hydrocarbon cracking.[7]
Cracking
Commercially, the dominant alkyne is acetylene itself, which is used as a fuel and a precursor to other compounds, e.g., acrylates. Hundreds of millions of kilograms are produced annually by partial oxidation of natural gas:[8]
Propyne, also industrially useful, is also prepared by thermal cracking of hydrocarbons.
Alkylation and arylation of terminal alkynes
Terminal alkynes (RC≡CH, including acetylene itself) can be deprotonated by bases like NaNH2, BuLi, or EtMgBr to give acetylide anions (RC≡C:–M+, M = Na, Li, MgBr) which can be alkylated by addition to carbonyl groups (Favorskii reaction), ring opening of epoxides, or SN2-type substitution of unhindered primary alkyl halides.
In the presence of transition metal catalysts, classically a combination of Pd(PPh3)2Cl2 and CuI, terminal acetylenes (RC≡CH) can react with aryl iodides and bromides (ArI or ArBr) in the presence of a secondary or tertiary amine like Et3N to give arylacetylenes (RC≡CAr) in the Sonogashira reaction.
The availability of these reliable reactions makes terminal alkynes useful building blocks for preparing internal alkynes.
Dehydrohalogenation and related reactions
Alkynes are prepared from 1,1- and 1,2-dihaloalkanes by double dehydrohalogenation. The reaction provides a means to generate alkynes from alkenes, which are first halogenated and then dehydrohalogenated. For example, phenylacetylene can be generated from styrene by bromination followed by treatment of the resulting of 1,2-dibromo-1-phenylethane with sodium amide in ammonia:[9][10]
- 350px
Via the Fritsch–Buttenberg–Wiechell rearrangement, alkynes are prepared from vinyl bromides. Alkynes can be prepared from aldehydes using the Corey–Fuchs reaction and from aldehydes or ketones by the Seyferth–Gilbert homologation.
Vinyl halides are susceptible to dehydrohalogenation.
Reactions, including applications
Featuring a reactive functional group, alkynes participate in many organic reactions. Such use was pioneered by Ralph Raphael, who in 1955 wrote the first book describing their versatility as intermediates in synthesis.[11] In spite of their kinetic stability (persistence) due to their strong triple bonds, alkynes are a thermodynamically unstable functional group, as can be gleaned from the highly positive heats of formation of small alkynes. For example, acetylene has a heat of formation of +227.4 kJ/mol (+54.2 kcal/mol), indicating a much higher energy content compared to its constituent elements. The highly exothermic combustion of acetylene is exploited industrially in oxyacetylene torches used in welding. Other reactions involving alkynes are often highly thermodynamically favorable (exothermic/exergonic) for the same reason.
Hydrogenation
Being more unsaturated than alkenes, alkynes characteristically undergo reactions that show that they are "doubly unsaturated". Alkynes are capable of adding two equivalents of H
2, whereas an alkene adds only one equivalent.[12] Depending on catalysts and conditions, alkynes add one or two equivalents of hydrogen. Partial hydrogenation, stopping after the addition of only one equivalent to give the alkene, is usually more desirable since alkanes are less useful:
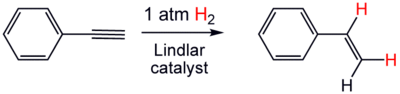
The largest scale application of this technology is the conversion of acetylene to ethylene in refineries (the steam cracking of alkanes yields a few percent acetylene, which is selectively hydrogenated in the presence of a palladium/silver catalyst). For more complex alkynes, the Lindlar catalyst is widely recommended to avoid formation of the alkane, for example in the conversion of phenylacetylene to styrene.[13] Similarly, halogenation of alkynes gives the alkene dihalides or alkyl tetrahalides:
The addition of one equivalent of H
2 to internal alkynes gives cis-alkenes.
Addition of halogens and related reagents
Alkynes characteristically are capable of adding two equivalents of halogens and hydrogen halides.
The addition of nonpolar E–H bonds across C≡C is general for silanes, boranes, and related hydrides. The hydroboration of alkynes gives vinylic boranes which oxidize to the corresponding aldehyde or ketone. In the thiol-yne reaction the substrate is a thiol.
Addition of hydrogen halides has long been of interest. In the presence of mercuric chloride as a catalyst, acetylene and hydrogen chloride react to give vinyl chloride. While this method has been abandoned in the West, it remains the main production method in China.[14]
Hydration
The hydration reaction of acetylene gives acetaldehyde. The reaction proceeds by formation of vinyl alcohol, which tautomerizes to form the aldehyde. This reaction was once a major industrial process but it has been displaced by the Wacker process. This reaction occurs in nature, the catalyst being acetylene hydratase.
Hydration of phenylacetylene gives acetophenone:[15]
(Ph
3P)AuCH
3 catalyzes hydration of 1,8-nonadiyne to 2,8-nonanedione:[16]
Isomerization to allenes
Alkynes can be isomerized by strong base or transition metals to allenes. Due to their comparable thermodynamic stabilities, the equilibrium constant of alkyne/allene isomerization is generally within several orders of magnitude of unity. For example propyne can be isomerized to give an equilibrium mixture with propadiene:
Cycloadditions and oxidation
Alkynes undergo diverse cycloaddition reactions. The Diels–Alder reaction with 1,3-dienes gives 1,4-cyclohexadienes. This general reaction has been extensively developed. Electrophilic alkynes are especially effective dienophiles. The "cycloadduct" derived from the addition of alkynes to 2-pyrone eliminates carbon dioxide to give the aromatic compound. Other specialized cycloadditions include multicomponent reactions such as alkyne trimerisation to give aromatic compounds and the [2+2+1]-cycloaddition of an alkyne, alkene and carbon monoxide in the Pauson–Khand reaction. Non-carbon reagents also undergo cyclization, e.g. azide alkyne Huisgen cycloaddition to give triazoles. Cycloaddition processes involving alkynes are often catalyzed by metals, e.g. enyne metathesis and alkyne metathesis, which allows the scrambling of carbyne (RC) centers:
Oxidative cleavage of alkynes proceeds via cycloaddition to metal oxides. Most famously, potassium permanganate converts alkynes to a pair of carboxylic acids.
Reactions specific for terminal alkynes
Terminal alkynes are readily converted to many derivatives, e.g. by coupling reactions and condensations. Via the condensation with formaldehyde and acetylene is produced butynediol:[8][17]
In the Sonogashira reaction, terminal alkynes are coupled with aryl or vinyl halides:
This reactivity exploits the fact that terminal alkynes are weak acids, whose typical pKa values around 25 place them between that of ammonia (35) and ethanol (16):
where MX = NaNH2, LiBu, or RMgX.
The reactions of alkynes with certain metal cations, e.g. Ag+
and Cu+
also gives acetylides. Thus, few drops of diamminesilver(I) hydroxide (Ag(NH
3)
2OH) reacts with terminal alkynes signaled by formation of a white precipitate of the silver acetylide. This reactivity is the basis of alkyne coupling reactions, including the Cadiot–Chodkiewicz coupling, Glaser coupling, and the Eglinton coupling shown below:[18]
In the Favorskii reaction and in alkynylations in general, terminal alkynes add to carbonyl compounds to give the hydroxyalkyne.
Metal complexes
Alkynes form complexes with transition metals. Such complexes occur also in metal catalyzed reactions of alkynes such as alkyne trimerization. Terminal alkynes, including acetylene itself, react with water to give aldehydes. The transformation typically requires metal catalysts to give this anti-Markovnikov addition result.[19]
Alkynes in nature and medicine
According to Ferdinand Bohlmann, the first naturally occurring acetylenic compound, dehydromatricaria ester, was isolated from an Artemisia species in 1826. In the nearly two centuries that have followed, well over a thousand naturally occurring acetylenes have been discovered and reported. Polyynes, a subset of this class of natural products, have been isolated from a wide variety of plant species, cultures of higher fungi, bacteria, marine sponges, and corals.[20] Some acids like tariric acid contain an alkyne group. Diynes and triynes, species with the linkage RC≡C–C≡CR′ and RC≡C–C≡C–C≡CR′ respectively, occur in certain plants (Ichthyothere, Chrysanthemum, Cicuta, Oenanthe and other members of the Asteraceae and Apiaceae families). Some examples are cicutoxin, oenanthotoxin, and falcarinol. These compounds are highly bioactive, e.g. as nematocides.[21] 1-Phenylhepta-1,3,5-triyne is illustrative of a naturally occurring triyne. Biosynthetically, the enediyne natural products are also derived from a polyyne precursor.
Alkynes occur in some pharmaceuticals, including the contraceptive noretynodrel. A carbon–carbon triple bond is also present in marketed drugs such as the antiretroviral efavirenz and the antifungal terbinafine. Molecules called ene-diynes feature a ring containing an alkene ("ene") between two alkyne groups ("diyne"). These compounds, e.g. calicheamicin, are some of the most aggressive antitumor drugs known, so much so that the ene-diyne subunit is sometimes referred to as a "warhead". Ene-diynes undergo rearrangement via the Bergman cyclization, generating highly reactive radical intermediates that attack DNA within the tumor.[22]
See also
References
- ↑ Alkyne. Encyclopædia Britannica
- ↑ Saul Patai, ed (1978). The Carbon–Carbon Triple Bond. 1. John Wiley & Sons. ISBN 9780470771563.
- ↑ Smith, Michael B.; March, Jerry (2006). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. p. 24. doi:10.1002/0470084960. ISBN 9780470084960.
- ↑ K. N. Campbell, B. K. Campbell (1950). "n-Butylacetylene". Organic Syntheses 30: 15. doi:10.15227/orgsyn.030.0015.
- ↑ Bloch, Daniel R. (2012). Organic Chemistry Demystified (2nd ed.). McGraw-Hill. pp. 57. ISBN 978-0-07-176797-2.
- ↑ The Commission on the Nomenclature of Organic Chemistry (1971). Nomenclature of Organic Chemistry (3rd ed.). London: Butterworths. ISBN 0-408-70144-7.
- ↑ Trotuş, Ioan-Teodor; Zimmermann, Tobias; Schüth, Ferdi (2014-02-12). "Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited". Chemical Reviews 114 (3): 1761–1782. doi:10.1021/cr400357r. ISSN 0009-2665. PMID 24228942. https://pubs.acs.org/doi/10.1021/cr400357r.
- ↑ 8.0 8.1 Gräfje, Heinz; Körnig, Wolfgang; Weitz, Hans-Martin; Reiß, Wolfgang; Steffan, Guido; Diehl, Herbert; Bosche, Horst; Schneider, Kurt et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_455.
- ↑ Kenneth N. Campbell, Barbara K. Campbell (1950). "Phenylacetylene". Organic Syntheses 30: 72. doi:10.15227/orgsyn.030.0072.
- ↑ A. Le Coq and A. Gorgues (1979). "Alkyness via Phase Transfer-Catalyzed Dehydrohalogenatiion: Propiolaldehyde Diethyl Acetal". Organic Syntheses 59: 10. doi:10.15227/orgsyn.059.0010.
- ↑ Raphael, Ralph Alexander (1955). Acetylenic compounds in organic synthesis. London: Butterworths Scientific Publications. OCLC 3134811. https://babel.hathitrust.org/cgi/pt?id=mdp.39015064396958;view=1up;seq=12.
- ↑ Rosser; Williams (1977). Modern Organic Chemistry for A-level. Great Britain: Collins. p. 82. ISBN 0003277402.
- ↑ H. Lindlar; R. Dubuis (1973). "Palladium catalyst for partial reduction of acetylenes". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv5p0880.; Collective Volume, 5, pp. 880.
- ↑ Dreher, Eberhard-Ludwig; Torkelson, Theodore R.; Beutel, Klaus K. (2011). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o01.
- ↑ Fukuda, Y.; Utimoto, K. (1991). "Effective transformation of unactivated alkynes into ketones or acetals with a gold(III) catalyst". J. Org. Chem. 56 (11): 3729. doi:10.1021/jo00011a058.
- ↑ Mizushima, E.; Cui, D.-M.; Nath, D. C. D.; Hayashi, T.; Tanaka, M. (2005). "Au(I)-Catalyzed hydratation of alkynes: 2,8-nonanedione". Organic Syntheses 83: 55. http://www.orgsyn.org/demo.aspx?prep=v83p0055.
- ↑ Peter Pässler; Werner Hefner; Klaus Buckl; Helmut Meinass; Andreas Meiswinkel; Hans-Jürgen Wernicke; Günter Ebersberg; Richard Müller et al. (2008). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_097.pub3.
- ↑ K. Stöckel and F. Sondheimer (1974). "[18]Annulene". Organic Syntheses 54: 1. doi:10.15227/orgsyn.054.0001.
- ↑ Hintermann, Lukas; Labonne, Aurélie (2007). "Catalytic Hydration of Alkynes and Its Application in Synthesis". Synthesis 2007 (8): 1121–1150. doi:10.1055/s-2007-966002.
- ↑ Annabelle L. K. Shi Shun; Rik R. Tykwinski (2006). "Synthesis of Naturally Occurring Polyynes". Angew. Chem. Int. Ed. 45 (7): 1034–1057. doi:10.1002/anie.200502071. PMID 16447152.
- ↑ Lam, Jørgen (1988). Chemistry and biology of naturally-occurring acetylenes and related compounds (NOARC): proceedings of a Conference on the Chemistry and Biology of Naturally-Occurring Acetylenes and Related Compounds (NOARC). Amsterdam: Elsevier. ISBN 0-444-87115-2.
- ↑ S. Walker; R. Landovitz; W.D. Ding; G.A. Ellestad; D. Kahne (1992). "Cleavage behavior of calicheamicin gamma 1 and calicheamicin T". Proc Natl Acad Sci USA 89 (10): 4608–12. doi:10.1073/pnas.89.10.4608. PMID 1584797. Bibcode: 1992PNAS...89.4608W.
 |