Chemistry:Nucleocidin
| |
| Names | |
|---|---|
| IUPAC name
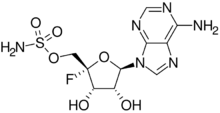
4′-Fluoroadenosine 5′-sulfamate
| |
| Systematic IUPAC name
[(2S,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-2-fluoro-3,4-dihydroxyoxan-2-yl]methyl sulfamate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H13FN6O6S | |
| Molar mass | 364.31 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nucleocidin is a fluorine-containing nucleoside produced by Streptomyces calvus.[1]
Chemical structure
Nucleocidin stems from the ribonucleoside adenosine[2] - is unique because it possess two rare functional groups: a fluorine atom and a sulfamyl ester [3]
During 1968 the attempts to identify nucleocidin were made and at that time it was assigned to a structure of a 9-adenyl-4' -sulfamoyloxypentofuranoside, which was mainly based on experiments from PMR and mass spectroscopy, as well as testing in chemical reactions. It was ultimately proven to have a structure of a 4' -fluoro-5' -O-sulphamoyladenosine.[4]
Microbiological origin
Nucleocidin is an antibiotic produced from Streptomyces calvus. Though toxic to mammals, it is able to function against bacteria both gram negative gram positive. It may be used against trypanosomes.[5]
Though commonly known to be produced by Streptomyces calvus, nucleocidin is produced in greater yield by Streptomyces virens and Streptomyces aurorectus.[6]
Biochemical relevance
The compound is capable of interrupting the synthesis of peptides.[7]
See also
References
- ↑ Bartholomé, Axel; Janso, Jeffrey E; Reilly, Usa; O'Hagan, David (2017). "Fluorometabolite biosynthesis: isotopically labelled glycerol incorporations into the antibiotic nucleocidin in Streptomyces calvus". Organic & Biomolecular Chemistry 15 (1): 61–64. doi:10.1039/c6ob02291j. PMID 27845468.
- ↑ Pasternak, Ola; Zechel, David (May 2022). "Identification of Genes Essential for Sulfamate and Fluorine Incorporation During Nucleocidin Biosynthesis" (in en). The FASEB Journal 36 (S1): fasebj.2022.36.S1.R2700. doi:10.1096/fasebj.2022.36.S1.R2700. ISSN 0892-6638. PMID 35552629. https://onlinelibrary.wiley.com/doi/10.1096/fasebj.2022.36.S1.R2700.
- ↑ Carvalho, Maria F.; Oliveira, Rui S. (2017-10-03). "Natural production of fluorinated compounds and biotechnological prospects of the fluorinase enzyme" (in en). Critical Reviews in Biotechnology 37 (7): 880–897. doi:10.1080/07388551.2016.1267109. ISSN 0738-8551. PMID 28049355. https://www.tandfonline.com/doi/full/10.1080/07388551.2016.1267109.
- ↑ Shuman, Dennis A.; Robins, Morris J.; Robins, Roland K. (June 1970). "Synthesis of Nucleoside Sulfamates Related to Nucleocidin". Journal of the American Chemical Society 92 (11): 3434–3440. doi:10.1021/ja00714a035. ISSN 0002-7863. PMID 5422764.
- ↑ Carvalho, Maria F.; Oliveira, Rui S (October 2017). "Natural Production of Fluorinated Compounds and Biotechnological Prospects of the Fluorinate Enzyme". Critical Reviews in Biotechnology 37 (7): 880–897. doi:10.1080/07388551.2016.1267109. ISSN 0738-8551. PMID 28049355.
- ↑ Pasternak, Ola; Zechel, David (May 2022). "Identification of Genes Essential for Sulfamate and Fluorine Incorporation During Nucleocidin Biosynthesis" (in en). The FASEB Journal 36 (S1): fasebj.2022.36.S1.R2700. doi:10.1096/fasebj.2022.36.S1.R2700. ISSN 0892-6638. PMID 35552629. https://onlinelibrary.wiley.com/doi/10.1096/fasebj.2022.36.S1.R2700.
- ↑ Bloch, A.; Coutsogeorgopoulos, C. (1971-11-01). "Inhibition of protein synthesis by 5'-sulfamoyladenosine" (in en). Biochemistry 10 (24): 4394–4398. doi:10.1021/bi00800a007. ISSN 0006-2960. PMID 4946919. https://pubs.acs.org/doi/abs/10.1021/bi00800a007.
 |
