Chemistry:Adenosine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Adenocard; Adenocor; Adenic; Adenoco; Adeno-Jec; Adenoscan; Adenosin; Adrekar; Krenosin |
| Other names | SR-96225 (developmental code name) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
(adenosine may be safe to the fetus in pregnant women) |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Rapidly cleared from circulation via cellular uptake |
| Protein binding | No |
| Metabolism | Rapidly converted to inosine and adenosine monophosphate |
| Elimination half-life | cleared plasma <30 seconds; half-life <10 seconds |
| Excretion | can leave cell intact or can be degraded to hypoxanthine, xanthine, and ultimately uric acid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H13N5O4 |
| Molar mass | 267.245 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
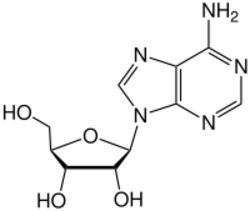
Adenosine (symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life on earth. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias.
Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12[1]) and as a radical in the radical SAM enzymes.[2]
Medical uses
Supraventricular tachycardia
In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert the rhythm.[3][4][5]
Certain SVTs can be successfully terminated with adenosine.[6] This includes any re-entrant arrhythmias that require the AV node for the re-entry, e.g., AV reentrant tachycardia (AVRT) and AV nodal reentrant tachycardia (AVNRT). In addition, atrial tachycardia can sometimes be terminated with adenosine.[7]
Fast rhythms of the heart that are confined to the atria (e.g., atrial fibrillation and atrial flutter) or ventricles (e.g., monomorphic ventricular tachycardia), and do not involve the AV node as part of the re-entrant circuit, are not typically converted by adenosine. However, the ventricular response rate is temporarily slowed with adenosine in such cases.[7]
Because of the effects of adenosine on AV node-dependent SVTs, adenosine is considered a class V antiarrhythmic agent. When adenosine is used to cardiovert an abnormal rhythm, it is normal for the heart to enter ventricular asystole for a few seconds. This can be disconcerting to a normally conscious patient, and is associated with angina-like sensations in the chest.[8]
Nuclear stress test
Adenosine is used as an adjunct to thallium (TI 201) or technetium (Tc99m) myocardial perfusion scintigraphy (nuclear stress test) in patients unable to undergo adequate stress testing with exercise.[9]
Dosage
When given for the evaluation or treatment of a supraventricular tachycardia (SVT), the initial dose is 6 mg to 12 mg, depending on standing orders or provider preference,[10] given as a rapid parenteral infusion. Due to adenosine's extremely short half-life, the IV line is started as proximal (near) to the heart as possible, such as the antecubital fossa. The IV push is often followed with a flush of 10–20 mL of normal saline. If this has no effect (i.e., no evidence of transient AV block), a dose of 12 mg can be given 1–2 minutes after the first dose. When given to dilate the arteries, such as in a "stress test", the dosage is typically 0.14 mg/kg/min, administered for 4 or 6 minutes, depending on the protocol.
The recommended dose may be increased in patients on theophylline since methylxanthines prevent binding of adenosine at receptor sites. The dose is often decreased in patients on dipyridamole (Persantine) and diazepam (Valium) because adenosine potentiates the effects of these drugs. The recommended dose is also reduced by half in patients presenting congestive heart failure, myocardial infarction, shock, hypoxia, and/or chronic liver disease or chronic kidney disease, and in elderly patients.
Drug interactions
Dipyridamole potentiates the action of adenosine, requiring the use of lower doses.

Methylxanthines (e.g. caffeine found in coffee, theophylline found in tea, or theobromine found in chocolate) have a purine structure and bind to some of the same receptors as adenosine.[12] Methylxanthines act as competitive antagonists of adenosine and can blunt its pharmacological effects.[13] Individuals taking large quantities of methylxanthines may require increased doses of adenosine.
Caffeine acts by blocking binding of adenosine to the adenosine A1 receptor, which enhances release of the neurotransmitter acetylcholine.[14] Caffeine also increases cyclic AMP levels through nonselective inhibition of phosphodiesterase.[15] "Caffeine has a three-dimensional structure similar to that of adenosine," which allows it to bind and block its receptors.[16]
Contraindications
Common contraindications for adenosine include
- Asthma, traditionally considered an absolute contraindication. This is being contested, and it is now considered a relative contraindication (however, selective adenosine antagonists are being investigated for use in treatment of asthma)[17]
Pharmacological effects
Adenosine is an endogenous purine nucleoside that modulates many physiological processes. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes (A1, A2A, A2B, and A3).[18]
Extracellular adenosine concentrations from normal cells are approximately 300 nM; however, in response to cellular damage (e.g., in inflammatory or ischemic tissue), these concentrations are quickly elevated (600–1,200 nM). Thus, in regard to stress or injury, the function of adenosine is primarily that of cytoprotection preventing tissue damage during instances of hypoxia, ischemia, and seizure activity. Activation of A2A receptors produces a constellation of responses that in general can be classified as anti-inflammatory.[19] Enzymatic production of adenosine can be anti-inflammatory or immunosuppressive.[20][21][22]
Adenosine receptors
All adenosine receptor subtypes (A1, A2A, A2B, and A3) are G-protein-coupled receptors. The four receptor subtypes are further classified based on their ability to either stimulate or inhibit adenylate cyclase activity. The A1 receptors couple to Gi/o and decrease cAMP levels, while the A2 adenosine receptors couple to Gs, which stimulates adenylate cyclase activity. In addition, A1 receptors couple to Go, which has been reported to mediate adenosine inhibition of Ca2+ conductance, whereas A2B and A3 receptors also couple to Gq and stimulate phospholipase activity. Researchers at Cornell University have recently shown adenosine receptors to be key in opening the blood-brain barrier (BBB). Mice dosed with adenosine have shown increased transport across the BBB of amyloid plaque antibodies and prodrugs associated with Parkinson's disease, Alzheimer's, multiple sclerosis, and cancers of the central nervous system.[23]
Ghrelin/growth hormone secretagogue receptor
Adenosine is an endogenous agonist of the ghrelin/growth hormone secretagogue receptor.[24] However, while it is able to increase appetite, unlike other agonists of this receptor, adenosine is unable to induce the secretion of growth hormone and increase its plasma levels.[24]
Mechanism of action
When it is administered intravenously, adenosine causes transient heart block in the atrioventricular (AV) node. This is mediated via the A1 receptor, inhibiting adenylyl cyclase, reducing cAMP and so causing cell hyperpolarization by increasing K+ efflux via inward rectifier K+ channels, subsequently inhibiting Ca2+ current.[25][26] It also causes endothelial-dependent relaxation of smooth muscle as is found inside the artery walls. This causes dilation of the "normal" segments of arteries, i.e. where the endothelium is not separated from the tunica media by atherosclerotic plaque. This feature allows physicians to use adenosine to test for blockages in the coronary arteries, by exaggerating the difference between the normal and abnormal segments.
The administration of adenosine also reduces blood flow to coronary arteries past the occlusion. Other coronary arteries dilate when adenosine is administered while the segment past the occlusion is already maximally dilated, which is a process called coronary steal. This leads to less blood reaching the ischemic tissue, which in turn produces the characteristic chest pain.
Metabolism
Adenosine used as a second messenger can be the result of de novo purine biosynthesis via adenosine monophosphate (AMP), though it is possible other pathways exist.[27]
When adenosine enters the circulation, it is broken down by adenosine deaminase, which is present in red blood cells and the vessel wall.
Dipyridamole, an inhibitor of adenosine nucleoside transporter, allows adenosine to accumulate in the blood stream. This causes an increase in coronary vasodilatation.
Adenosine deaminase deficiency is a known cause of immunodeficiency.
Research
Viruses
The adenosine analog NITD008 has been reported to directly inhibit the recombinant RNA-dependent RNA polymerase of the dengue virus by terminating its RNA chain synthesis. This interaction suppresses peak viremia and rise in cytokines and prevents lethality in infected animals, raising the possibility of a new treatment for this flavivirus.[28] The 7-deaza-adenosine analog has been shown to inhibit the replication of the hepatitis C virus.[29] BCX4430 is protective against Ebola and Marburg viruses.[30] Such adenosine analogs are potentially clinically useful since they can be taken orally.
Anti-inflammatory properties
Adenosine is believed to be an anti-inflammatory agent at the A2A receptor.[31][32] Topical treatment of adenosine to foot wounds in diabetes mellitus has been shown in lab animals to drastically increase tissue repair and reconstruction. Topical administration of adenosine for use in wound-healing deficiencies and diabetes mellitus in humans is currently under clinical investigation.
Methotrexate's anti-inflammatory effect may be due to its stimulation of adenosine release.[33]
Central nervous system
In general, adenosine has an inhibitory effect in the central nervous system (CNS). Caffeine's stimulatory effects are credited primarily (although not entirely) to its capacity to block adenosine receptors, thereby reducing the inhibitory tonus of adenosine in the CNS. This reduction in adenosine activity leads to increased activity of the neurotransmitters dopamine and glutamate.[34] Experimental evidence suggests that adenosine and adenosine agonists can activate Trk receptor phosphorylation through a mechanism that requires the adenosine A2A receptor.[35]
Hair
Adenosine has been shown to promote thickening of hair on people with thinning hair.[36][37] A 2013 study compared topical adenosine with minoxidil in male androgenetic alopecia, finding it was as potent as minoxidil (in overall treatment outcomes) but with higher satisfaction rate with patients due to “faster prevention of hair loss and appearance of the newly grown hairs” (further trials were called for to clarify the findings).[38]
Sleep
Adenosine is a key factor in regulating the body's sleep-wake cycle.[39] Adenosine levels rise during periods of wakefulness and lowers during sleep. Higher adenosine levels correlate with a stronger feeling of sleepiness, also known as sleep drive or sleep pressure.[40] Cognitive behavioral therapy for insomnia (CBT-I), which is considered one of the most effective treatments for insomnia, utilizes short-term sleep deprivation to raise and regulate adenosine levels in the body, for the intended promotion of consistent and sustained sleep in the long term.[41]
A principal component of cannabis delta-9-tetrahydrocannabinol (THC) and the endocannabinoid anandamide (AEA) induces sleep in rats by increasing adenosine levels in the basal forebrain. These components also significantly increase slow-wave sleep during the sleep cycle, mediated by CB1 receptor activation. These findings identify a potential therapeutic use of cannabinoids to induce sleep in conditions where sleep may be severely attenuated.[42]
Vasodilation
It also plays a role in regulation of blood flow to various organs through vasodilation.[43][44][45]
See also
- Adenosine receptor
- Adenosine reuptake inhibitor
- List of growth hormone secretagogues
References
- ↑ "Biological Organometallic Chemistry of B12". Bioorganometallic Chemistry. Topics in Organometallic Chemistry. 17. 2006. pp. 1–55. doi:10.1007/3418_004. ISBN 3-540-33047-X.
- ↑ Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, ISBN 0-7167-4339-6
- ↑ "Pharmacology of Adenosine Receptors: The State of the Art". Physiological Reviews 98 (3): 1591–1625. July 2018. doi:10.1152/physrev.00049.2017. PMID 29848236.
- ↑ "Clinical practice. Supraventricular tachycardia". The New England Journal of Medicine 354 (10): 1039–1051. March 2006. doi:10.1056/NEJMcp051145. PMID 16525141.
- ↑ "Electrophysiologic effects of adenosine triphosphate and adenosine on the mammalian heart: clinical and experimental aspects". Journal of the American College of Cardiology 4 (2): 414–424. August 1984. doi:10.1016/S0735-1097(84)80233-8. PMID 6376597.
- ↑ "Wide QRS complex tachycardia. Diagnosis: Supraventricular tachycardia with aberrant conduction; intravenous (IV) adenosine". CJEM 10 (6): 572–3, 581. November 2008. PMID 19000353.
- ↑ 7.0 7.1 "Reentry Arrhythmia". StatPearls. Treasure Island, FL: StatPearls Publishing. 2022. http://www.ncbi.nlm.nih.gov/books/NBK537089/. Retrieved 2022-01-28.
- ↑ Coronary Pressure. Springer. 2000. ISBN 0-7923-6170-9.[page needed]
- ↑ "Safety and diagnostic accuracy of adenosine thallium-201 scintigraphy in patients unable to exercise and those with left bundle branch block". American Heart Journal 124 (3): 614–621. September 1992. doi:10.1016/0002-8703(92)90268-z. PMID 1514488.
- ↑ "2014 Guidelines". April 2016. http://www.regionsems.com/wp-content/uploads/2016/04/2014-Guidelines.pdf.
- ↑ "Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension". Pulmonary Circulation 10 (1): 432–439. 2005. doi:10.1192/apt.11.6.432. PMID 32166015.
- ↑ "Caffeine and adenosine". Journal of Alzheimer's Disease 20 (Suppl 1): S3-15. 2010. doi:10.3233/JAD-2010-1379. PMID 20164566.
- ↑ "Vitamin B4". R&S Pharmchem. April 2011. http://www.rspharmchem.com/vitamin-b4.htm.
- ↑ "Caffeine enhances acetylcholine release in the hippocampus in vivo by a selective interaction with adenosine A1 receptors". The Journal of Pharmacology and Experimental Therapeutics 273 (2): 637–642. May 1995. PMID 7752065.
- ↑ "The Medicinal Chemistry of Caffeine". Journal of Medicinal Chemistry 64 (11): 7156–7178. June 2021. doi:10.1021/acs.jmedchem.1c00261. PMID 34019396.
- ↑ Principles of Life (2 ed.). Macmillan Learning. 2015. pp. 102–103. ISBN 978-1-4641-8652-3.
- ↑ "Adenosine receptors and asthma". British Journal of Pharmacology 153 (Suppl 1): S446–S456. March 2008. doi:10.1038/bjp.2008.22. PMID 18311158.
- ↑ "Adenosine receptors: therapeutic aspects for inflammatory and immune diseases". Nature Reviews. Drug Discovery 7 (9): 759–770. September 2008. doi:10.1038/nrd2638. PMID 18758473.
- ↑ "Adenosine: an endogenous regulator of innate immunity". Trends in Immunology 25 (1): 33–39. January 2004. doi:10.1016/j.it.2003.11.003. PMID 14698282.
- ↑ "Targeting Adenosine Receptor Signaling in Cancer Immunotherapy". International Journal of Molecular Sciences 19 (12): 3837. December 2018. doi:10.3390/ijms19123837. PMID 30513816.
- ↑ "The Good, the Bad and the Unknown of CD38 in the Metabolic Microenvironment and Immune Cell Functionality of Solid Tumors". Cells 9 (1): 52. December 2019. doi:10.3390/cells9010052. PMID 31878283.
- ↑ "CD39 and CD73 in immunity and inflammation". Trends in Molecular Medicine 19 (6): 355–367. June 2013. doi:10.1016/j.molmed.2013.03.005. PMID 23601906.
- ↑ "Adenosine receptor signaling modulates permeability of the blood-brain barrier". The Journal of Neuroscience 31 (37): 13272–13280. September 2011. doi:10.1523/JNEUROSCI.3337-11.2011. PMID 21917810.
- ↑ 24.0 24.1 "Role of the Growth Hormone Secretagogue Receptor in the Central Nervous System". Brain Somatic Cross-Talk and the Central Control of Metabolism. Springer Science & Business Media. 2012. pp. 42–. ISBN 978-3-642-18999-9. https://books.google.com/books?id=Ml7vCAAAQBAJ&pg=PA42.
- ↑ "Аденозин в косметике - Польза антивозрастной корейской косметики" (in ru-RU). 2021-03-18. https://kimito.com.ua/adenozin-v-kosmetike/.
- ↑ Basic & Clinical Pharmacology (12th ed.). McGraw Hill. 2012. p. 245. ISBN 978-0-07-176402-5.
- ↑ "Effects of the purine biosynthesis pathway inhibitors azaserine, hadacidin, and mycophenolic acid on the developing ovine corpus luteum". The Chinese Journal of Physiology 36 (4): 245–252. 1993. PMID 8020339.
- ↑ "An adenosine nucleoside inhibitor of dengue virus". Proceedings of the National Academy of Sciences of the United States of America 106 (48): 20435–20439. December 2009. doi:10.1073/pnas.0907010106. PMID 19918064. Bibcode: 2009PNAS..10620435Y.
- ↑ "A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties". Antimicrobial Agents and Chemotherapy 48 (10): 3944–3953. October 2004. doi:10.1128/AAC.48.10.3944-3953.2004. PMID 15388457.
- ↑ "Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430". Nature 508 (7496): 402–405. April 2014. doi:10.1038/nature13027. PMID 24590073. Bibcode: 2014Natur.508..402W.
- ↑ "Anti-inflammatory preconditioning by agonists of adenosine A1 receptor". PLOS ONE 3 (5): e2107. May 2008. doi:10.1371/journal.pone.0002107. PMID 18461129. Bibcode: 2008PLoSO...3.2107N.
- ↑ "Treating lung inflammation with agonists of the adenosine A2A receptor: promises, problems and potential solutions". British Journal of Pharmacology 155 (4): 463–474. October 2008. doi:10.1038/bjp.2008.329. PMID 18846036.
- ↑ "How does methotrexate suppress inflammation?". Clinical and Experimental Rheumatology 28 (5 Suppl 61): S21–S23. 2010. PMID 21044428.
- ↑ "Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens". The Journal of Neuroscience 22 (15): 6321–6324. August 2002. doi:10.1523/JNEUROSCI.22-15-06321.2002. PMID 12151508.
- ↑ "Activation of Trk neurotrophin receptors in the absence of neurotrophins". Proceedings of the National Academy of Sciences of the United States of America 98 (6): 3555–3560. March 2001. doi:10.1073/pnas.061020198. PMID 11248116. Bibcode: 2001PNAS...98.3555L.
- ↑ "Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: a pilot, double-blind, randomized, placebo-controlled trial". The Journal of Dermatology 35 (12): 763–767. December 2008. doi:10.1111/j.1346-8138.2008.00564.x. PMID 19239555.
- ↑ "Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles". International Journal of Molecular Medicine 29 (2): 195–201. February 2012. doi:10.3892/ijmm.2011.817. PMID 22020741.
- ↑ "Comparison of the efficacy of topical minoxidil 5% and adenosine 0.75% solutions on male androgenetic alopecia and measuring patient satisfaction rate". Acta Dermatovenerologica Croatica 21 (3): 155–159. 2013. PMID 24183218.
- ↑ "Adenosine, caffeine, and sleep-wake regulation: state of the science and perspectives". Journal of Sleep Research 31 (4): e13597. August 2022. doi:10.1111/jsr.13597. PMID 35575450.
- ↑ "Adenosine and Sleep" (in en-US). 2022-06-07. https://www.sleepfoundation.org/how-sleep-works/adenosine-and-sleep.
- ↑ "Models of insomnia.". Principles and Practice of Sleep Medicine. (Elsevier Inc.) 5 (1): 850–865. January 2011. doi:10.1016/B978-1-4160-6645-3.00078-5. ISBN 9781416066453. https://www.med.upenn.edu/cbti/assets/user-content/documents/ppsmmodelsofinsomnia20115theditionproof.pdf.
- ↑ "Anandamide enhances extracellular levels of adenosine and induces sleep: an in vivo microdialysis study". Sleep 26 (8): 943–947. December 2003. doi:10.1093/sleep/26.8.943. PMID 14746372.
- ↑ "Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease". American Journal of Physiology. Heart and Circulatory Physiology 288 (4): H1633–H1640. April 2005. doi:10.1152/ajpheart.00575.2004. PMID 15772334.
- ↑ "Role of nitric oxide in adenosine-induced vasodilation in humans". Hypertension 31 (5): 1061–1064. May 1998. doi:10.1161/01.HYP.31.5.1061. PMID 9576114.
- ↑ "Adenosine as a vasodilator in primary pulmonary hypertension". Circulation 84 (3): 1145–1149. September 1991. doi:10.1161/01.CIR.84.3.1145. PMID 1884445.
 |
