Chemistry:OX1001
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
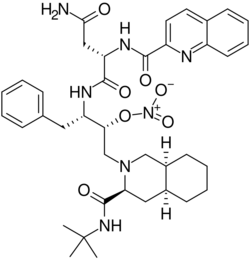
| Formula | C38H49N7O7 |
| Molar mass | 715.852 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
OX1001 (saquinavir-NO) is an experimental drug being developed by OncoNOx currently undergoing clinical studies and investigations for the treatment of cancer. OX1001 is a nitrate ester analog of the approved HIV protease inhibitor, saquinavir. This modification increases the anti-cancer property while decreasing toxicity of the drug. OX1001 shows broad activity against cancer cells but is particularly effective against hematological, prostate, and melanoma cancers as seen in in vitro and in vivo studies.[1]
Mechanism of action
OX1001 is an analog of the already approved HIV protease inhibitor, saquinavir. HIV protease inhibitor drugs not only work against HIV, but they also have proved function in anti-cancer therapy.[2] However, these drugs tend to have many toxic effects. Adding a nitrate ester functional group to HIV protease inhibitors has been found to curb these negative effects as well as increase the anti-tumor properties of the drug.[2][3]
Because OX1001 is still in pre-clinical testing, its mechanism of action is unclear. However, it can be said that as opposed to saquinavir, OX1001 likely works by slowing the growth rate of tumor cells rather than by causing apoptosis, or cell death.[2][3] Studies suggest that this stoppage of cell proliferation is permanent.[2]
Though the difference between OX1001 and saquinavir lies in the presence of a nitrate ester, it is not clear how exactly this modification directly affects the function of the drug.[2] It can be hypothesized that the presence of the nitrate ester plays a role in inhibiting the activity of cytochrome P450, which is an enzyme that normally deactivates saquinavir.[2]
Aside from having superior anti-tumor properties, OX1001 also has a much lower toxicity profile than saquinavir. In one experiment, it was determined that at a dose where Saquinavir produced 100% toxicity, OX1001 showed no signs of inducing any toxicity.[4] This might be due to the drug’s ability to produce a powerful, yet short-lived stimulus to normal cells’ Akt signaling pathways which protects them.[2]
References
- ↑ "OncoNOx". http://www.onconox.com/.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "The antitumor properties of a nontoxic, nitric oxide-modified version of saquinavir are independent of Akt". Molecular Cancer Therapeutics 8 (5): 1169–78. May 2009. doi:10.1158/1535-7163.MCT-08-0998. PMID 19417156.
- ↑ 3.0 3.1 "Nitric-oxide-donating NSAIDs as agents for cancer prevention". Trends in Molecular Medicine 10 (7): 324–30. July 2004. doi:10.1016/j.molmed.2004.05.004. PMID 15242680.
- ↑ "Anticancer effects of the nitric oxide-modified saquinavir derivative saquinavir-NO against multidrug-resistant cancer cells". Neoplasia 12 (12): 1023–30. December 2010. doi:10.1593/neo.10856. PMID 21170266.
Further reading
- "The new and less toxic protease inhibitor saquinavir-NO maintains anti-HIV-1 properties in vitro indistinguishable from those of the parental compound saquinavir". Antiviral Research 91 (3): 292–5. September 2011. doi:10.1016/j.antiviral.2011.07.001. PMID 21763726.
- "Cytotoxic and immune-sensitizing properties of nitric oxide-modified Saquinavir in iNOS-positive human melanoma cells". Journal of Cellular Physiology 226 (7): 1803–12. July 2011. doi:10.1002/jcp.22513. PMID 21506111.
 |

