Chemistry:Olverembatinib
From HandWiki
Short description: Chemical compound
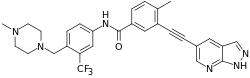 | |
| Clinical data | |
|---|---|
| Other names | GZD-824; GZD824 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H27F3N6O |
| Molar mass | 532.571 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Olverembatinib is a BCR-ABL1 tyrosine kinase inhibitor developed by Ascentage Pharma. In 2021, it was approved in China "for the treatment of adult patients with TKI-resistant chronic-phase CML (CML-CP) or accelerated-phase CML (CML-AP) harbouring the T315I mutation".[1][2][3]
References
- ↑ Dhillon, Sohita (March 2022). "Olverembatinib: First Approval". Drugs 82 (4): 469–475. doi:10.1007/s40265-022-01680-9. PMID 35195876. https://figshare.com/articles/online_resource/Olverembatinib_First_Approval/19111685.
- ↑ Jiang, Qian; Li, Zongru; Qin, Yazhen; Li, Weiming; Xu, Na; Liu, Bingcheng; Zhang, Yanli; Meng, Li et al. (18 August 2022). "Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: results of an open-label, multicenter phase 1/2 trial". Journal of Hematology & Oncology 15 (1): 113. doi:10.1186/s13045-022-01334-z. PMID 35982483.
- ↑ Jiang, Qian; Huang, Xiaojun; Chen, Zi; Niu, Qian; Shi, Dayu; Li, Zongru; Hou, Yue; Hu, Yu et al. (5 November 2020). "Novel BCR-ABL1 Tyrosine Kinase Inhibitor (TKI) HQP1351 (Olverembatinib) Is Efficacious and Well Tolerated in Patients with T315I-Mutated Chronic Myeloid Leukemia (CML): Results of Pivotal (Phase II) Trials". Blood 136 (Supplement 1): 50–51. doi:10.1182/blood-2020-142142.
 |

