Chemistry:Olympiadane
From HandWiki
| |
| Error creating thumbnail: Unable to save thumbnail to destination | |
| Names | |
|---|---|
| IUPAC name
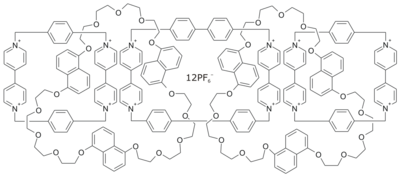
6,9,12,15,18,29,32,35,38,41,52,55,58,61,64-pentadecaoxaheptacyclo[63.4.0.05,69.019,24.023,28.042,47.046,51]nonahexaconta-1(69),2,4,19,21,23,25,27,42,44,46,48,50,65,67-pentadecaene;5,12,19,26-tetrazoniaheptacyclo[24.2.2.22,5.27,10.212,15.216,19.221,24]tetraconta-1(29),2(40),3,5(39),7,9,12,14,16(34),17,19(33),21(32),22,24(31),26(30),27,35,37-octadecaene;dodecahexafluorophosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C228H236F72N12O30P12 | |
| Molar mass | 5364.020 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Olympiadane is a mechanically interlocked molecule composed of five interlocking macrocycles that resembles the Olympic rings. The molecule is a linear pentacatenane or a [5]catenane. It was synthesized and named by Fraser Stoddart and coworkers in 1994.[1] The molecule was designed without any practical use in mind,[2] although other catenanes may have possible application to the construction of a molecular computer.
See also
References
- ↑ Amabilino, D. B.; Ashton, P. R.; Reder, A. S.; Spencer, N.; Stoddart, J. F. (1994). "Olympiadane". Angew. Chem. Int. Ed. Engl. 33 (12): 1286–1290. doi:10.1002/anie.199412861.
- ↑ Browne, M. W. (30 August 1994). "Chemists Make Rings Of Interlocked Atoms, A Clue to Life's Origin". The New York Times. https://www.nytimes.com/1994/08/30/science/chemists-make-rings-of-interlocked-atoms-a-clue-to-life-s-origin.html. Retrieved 3 January 2016.
 |
