Chemistry:Oosporein
From HandWiki
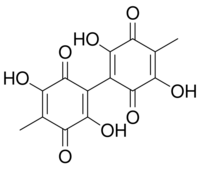
| |
| Names | |
|---|---|
| IUPAC name
2-(2,5-dihydroxy-4-methyl-3,6-dioxocyclohexa-1,4-dien-1-yl)-3,6-dihydroxy-5-methylcyclohexa-2,5-diene-1,4-dione
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H10O8 | |
| Molar mass | 306.226 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Oosporein is a toxic, bronze colored dibenzoquinone with the molecular formula C14H10O8.[2][3] Oosporein was first extracted from various molds and has antibiotic,[4] antiviral, cytotoxic, antifungal, and Insecticide properties.[3][5][2]
References
- ↑ (in en) Oosporein (CAS 475-54-7). https://www.caymanchem.com/product/29173.
- ↑ 2.0 2.1 2.2 2.3 Eisenbrand, Gerhard; Schreier, Peter (14 May 2014) (in de). RÖMPP Lexikon Lebensmittelchemie, 2. Auflage, 2006. Georg Thieme Verlag. p. 824. ISBN 978-3-13-179282-2.
- ↑ 3.0 3.1 Feng, Peng; Shang, Yanfang; Cen, Kai; Wang, Chengshu (8 September 2015). "Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity". Proceedings of the National Academy of Sciences 112 (36): 11365–11370. doi:10.1073/pnas.1503200112. PMID 26305932.
- ↑ (in en) Handbook of Applied Mycology: Volume 2: Humans, Animals and Insects. CRC Press. p. 626. ISBN 978-0-8247-8435-5.
- ↑ Ramesha, Alurappa; Venkataramana, M.; Nirmaladevi, Dhamodaran; Gupta, Vijai K.; Chandranayaka, S.; Srinivas, Chowdappa (1 September 2015). "Cytotoxic effects of oosporein isolated from endophytic fungus Cochliobolus kusanoi". Frontiers in Microbiology 6: 870. doi:10.3389/fmicb.2015.00870. PMID 26388840.
Further reading
- Mc Namara, Louise; Dolan, Stephen K.; Walsh, John M.D.; Stephens, John C.; Glare, Travis R.; Kavanagh, Kevin; Griffin, Christine T. (August 2019). "Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence". Fungal Biology 123 (8): 601–610. doi:10.1016/j.funbio.2019.01.004. PMID 31345414. http://mural.maynoothuniversity.ie/13591/1/CG_Oosporein.pdf.
- Kögl, F.; van Wessem, G. C. (1944). "Untersuchungen über Pilzfarbstoffe XIV : Über Oosporein, den Farbstoff von Oospora colorans van Beyma". Recueil des Travaux Chimiques des Pays-Bas 63 (1): 5–12. doi:10.1002/recl.19440630102.
- Ross, P. Frank; Osheim, David L.; Rottinghaus, George E. (July 1989). "Mass Spectral Confirmation of Oosporein in Poultry Rations". Journal of Veterinary Diagnostic Investigation 1 (3): 271–272. doi:10.1177/104063878900100317. PMID 2488354.
- (in en) Canadian Journal of Biochemistry. National Research Council of Canada. p. 562.
- Smith, DUPLICATE; Henderson, Rachel (23 July 1991) (in en). Mycotoxins and Animal Foods. CRC Press. p. 586. ISBN 978-0-8493-4904-1.
 |
