Biology:Insecticide


Insecticides are pesticides used to kill insects.[1] They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity.[2] Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain.
Insecticides can be classified into two major groups: systemic insecticides, which travel though the plant after uptake; and contact insecticides, which do not.[3]
The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals.
Insecticides may be repellent or non-repellent. Social insects such as ants cannot detect non-repellents and readily crawl through them. As they return to the nest they take insecticide with them and transfer it to their nestmates. Over time, this eliminates all of the ants including the queen. This is slower than some other methods, but usually completely eradicates the ant colony.[4]
Insecticides are distinct from non-insecticidal repellents, which repel but do not kill.
Type of activity
Systemic insecticides
Systemic insecticides, after uptake, are distributed systemically throughout the whole plant. When insects feed on the plant, they ingest the insecticide. Systemic insecticides produced by transgenic plants are called plant-incorporated protectants (PIPs). For instance, a gene that codes for a specific Bacillus thuringiensis biocidal protein was introduced into corn (maize) and other species. The plant manufactures the protein, which kills the insect when consumed.[5]
Contact insecticides
Contact insecticides are toxic to insects upon direct contact. These can be inorganic insecticides, which are metals and include the commonly used sulfur, and the less commonly used arsenates, copper and fluorine compounds. Contact insecticides can also be organic insecticides, i.e. organic chemical compounds, synthetically produced, and comprising the largest numbers of pesticides used today. Or they can be natural compounds like pyrethrum, neem oil, etc.
Efficacy can be related to the quality of pesticide application, with small droplets, such as aerosols often improving performance.[6]
Synthetic insecticides
Development
Organochlorides
The best known organochloride, DDT, was created by Swiss scientist Paul Müller. For this discovery, he was awarded the 1948 Nobel Prize for Physiology or Medicine.[7] DDT was introduced in 1944. It functions by opening sodium channels in the insect's nerve cells.[8] The contemporaneous rise of the chemical industry facilitated large-scale production of chlorinated hydrocarbons including various cyclodiene and hexachlorocyclohexane compounds.
Organophosphates
Organophosphates are another large class of contact insecticides. These also target the insect's nervous system. Organophosphates interfere with the enzymes acetylcholinesterase and other cholinesterases, causing an increase in synaptic acetylcholine and overstimulation of the parasympathetic nervous system.[9] and killing or disabling the insect. Organophosphate insecticides and chemical warfare nerve agents (such as sarin, tabun, soman, and VX) have the same mechanism of action. Organophosphates have a cumulative toxic effect to wildlife, so multiple exposures to the chemicals amplifies the toxicity.[10] In the US, organophosphate use declined with the rise of substitutes.[11] Many of these insecticides, first developed in the mid 20th century, are very poisonous. Although commonly used in the past, many older chemicals have been removed from the market due to their health and environmental effects (e.g. DDT, chlordane, and toxaphene).[12][13][14] Many organophosphates do not persist in the environment.
Carbamates
Carbamate insecticides have similar mechanisms to organophosphates, but have a much shorter duration of action and are somewhat less toxic.[citation needed]
Pyrethroids
Pyrethroid insecticides mimic the insecticidal activity of the natural compound pyrethrin, the biopesticide found in Pyrethrum (Now Chrysanthemum and Tanacetum) species. They have been modified to increase their stability in the environment. These compounds are nonpersistent sodium channel modulators and are less toxic than organophosphates and carbamates. Compounds in this group are often applied against household pests.[15] Some synthetic pyrethroids are toxic to the nervous system.[16]
Neonicotinoids
Neonicotinoids are a class of neuro-active insecticides chemically similar to nicotine.(with much lower acute mammalian toxicity and greater field persistence). These chemicals are acetylcholine receptor agonists. They are broad-spectrum systemic insecticides, with rapid action (minutes-hours). They are applied as sprays, drenches, seed and soil treatments. Treated insects exhibit leg tremors, rapid wing motion, stylet withdrawal (aphids), disoriented movement, paralysis and death.[17]Imidacloprid, of the neonicotinoid family, is the most widely used insecticide in the world.[18] In the late 1990s neonicotinoids came under increasing scrutiny over their environmental impact and were linked in a range of studies to adverse ecological effects, including honey-bee colony collapse disorder (CCD) and loss of birds due to a reduction in insect populations. In 2013, the European Union and a few non EU countries restricted the use of certain neonicotinoids.[19][20][21][22][23][24][25][26] and its potential to increase the susceptibility of rice to planthopper attacks.[27]
Phenylpyrazoles
Phenylpyrazole insecticides, such as fipronil are a class of synthetic insecticides that operate by interfering with GABA receptors.[28]
Butenolides
Butenolide pesticides are a novel group of chemicals, similar to neonicotinoids in their mode of action, that have so far only one representative: flupyradifurone. They are acetylcholine receptor agonists, like neonicotinoids, but with a different pharmacophore.[29] They are broad-spectrum systemic insecticides, applied as sprays, drenches, seed and soil treatments. Although the classic risk assessment considered this insecticide group (and flupyradifurone specifically) safe for bees, novel research[30] has raised concern on their lethal and sublethal effects, alone or in combination with other chemicals or environmental factors.[31][32]
Ryanoids/diamides
Diamides are synthetic ryanoid analogues with the same mode of action as ryanodine, a naturally occurring insecticide extracted from Ryania speciosa (Salicaceae). They bind to calcium channels in cardiac and skeletal muscle, blocking nerve transmission. The first insecticide from this class to be registered was Rynaxypyr, generic name chlorantraniliprole.[33]
Insect growth regulators
Insect growth regulator (IGR) is a term coined to include insect hormone mimics and an earlier class of chemicals, the benzoylphenyl ureas, which inhibit chitin (exoskeleton) biosynthesis in insects[34] Diflubenzuron is a member of the latter class, used primarily to control caterpillars that are pests. The most successful insecticides in this class are the juvenoids (juvenile hormone analogues). Of these, methoprene is most widely used. It has no observable acute toxicity in rats and is approved by World Health Organization (WHO) for use in drinking water cisterns to combat malaria. Most of its uses are to combat insects where the adult is the pest, including mosquitoes, several fly species, and fleas. Two very similar products, hydroprene and kinoprene, are used for controlling species such as cockroaches and white flies. Methoprene was registered with the EPA in 1975. Virtually no reports of resistance have been filed. A more recent type of IGR is the ecdysone agonist tebufenozide (MIMIC), which is used in forestry and other applications for control of caterpillars, which are far more sensitive to its hormonal effects than other insect orders.
Biological pesticides
More natural insecticides have been interesting targets of research for two main reasons, firstly because the most common chemicals are losing effectiveness, and secondly due to their toxic effects upon the environment.[35] Many organic compounds are already produced by plants for the purpose of defending the host plant from predation, and can be turned toward human ends.
Four extracts of plants are in commercial use: pyrethrum, rotenone, neem oil, and various essential oils[36]
A trivial case is tree rosin, which is a natural insecticide. Specifically, the production of oleoresin by conifer species is a component of the defense response against insect attack and fungal pathogen infection.[37] Many fragrances, e.g. oil of wintergreen, are in fact antifeedants.
Other biological approaches
Plant-incorporated protectants
Bacillus thuringiensis
Transgenic crops that act as insecticides began in 1996 with a genetically modified potato that produced Cry proteins, derived from the bacterium Bacillus thuringiensis, which is toxic to beetle larvae such as the Colorado potato beetle.[38]
RNA interference
The technique has been expanded to include the use of RNAi insecticides which fatally silence crucial insect genes. (RNAi likely originally evolved as a defense against viruses.)[38] This was first demonstrated by Baum et al. 2007, who incorporated a V-APTase as a protectant into transgenic Zea mays and demonstrated effectiveness against Diabrotica virgifera virgifera. This suggests oral delivery against Coleoptera as a whole will probably be effective. Similar studies have followed Baum's technique to protect with dsRNAs targeting detox, especially insect P450s. Bolognesi et al. 2012 is one of these following studies, however they found dsRNA to be processed into siRNAs by the plants (in this case Solanum tuberosum) themselves, and siRNAs to be less effectively taken up by insect cells. Bolognesi therefore produced additional transgenic S. tuberosum plants which instead produced longer dsRNAs in the chloroplasts, which naturally accumulate dsRNAs but do not have the machinery to convert them to siRNAs.[39] Midgut cells in many larvae take up the molecules and help spread the signal. The technology can target only insects that have the silenced sequence, as was demonstrated when a particular RNAi affected only one of four fruit fly species. The technique is expected to replace many other insecticides,[dubious ] which are losing effectiveness due to the spread of insecticide resistance.[38]
Venom
Spider venom peptide fractions are another class of potential transgenic traits which could expand the mode of action repertoire and help to answer the resistance question.[40]
Enzymes
Many plants exude substances to repel insects. Premier examples are substances activated by the enzyme myrosinase. This enzyme converts glucosinolates to various compounds that are toxic to herbivorous insects. One product of this enzyme is allyl isothiocyanate, the pungent ingredient in horseradish sauces.
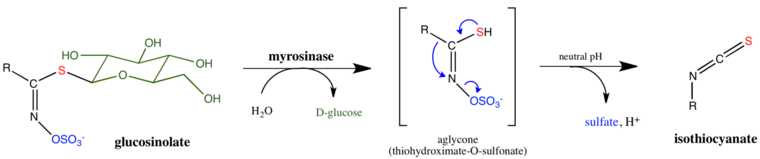
The myrosinase is released only upon crushing the flesh of horseradish. Since allyl isothiocyanate is harmful to the plant as well as the insect, it is stored in the harmless form of the glucosinolate, separate from the myrosinase enzyme.[41]
Bacterial
Bacillus thuringiensis is a bacterial disease that affects Lepidopterans and some other insects. Toxins produced by strains of this bacterium are used as a larvicide against caterpillars, beetles, and mosquitoes. Toxins from Saccharopolyspora spinosa are isolated from fermentations and sold as Spinosad. Because these toxins have little effect on other organisms, they are considered more environmentally friendly than synthetic pesticides. The toxin from B. thuringiensis (Bt toxin) has been incorporated directly into plants through the use of genetic engineering.
Other
Other biological insecticides include products based on entomopathogenic fungi (e.g., Beauveria bassiana, Metarhizium anisopliae), nematodes (e.g., Steinernema feltiae) and viruses (e.g., Cydia pomonella granulovirus).[citation needed]
Synthetic insecticide and natural insecticides
A major emphasis of organic chemistry is the development of chemical tools to enhance agricultural productivity. Insecticides represent a major area of emphasis. Many of the major insecticides are inspired by biological analogues. Many others are not found in nature.
Environmental harm
Effects on nontarget species
Some insecticides kill or harm other creatures in addition to those they are intended to kill. For example, birds may be poisoned when they eat food that was recently sprayed with insecticides or when they mistake an insecticide granule on the ground for food and eat it.[10] Sprayed insecticide may drift from the area to which it is applied and into wildlife areas, especially when it is sprayed aerially.[10]
DDT
The development of DDT was motivated by desire to replace more dangerous or less effective alternatives. DDT was introduced to replace lead and arsenic-based compounds, which were in widespread use in the early 1940s.[42]
DDT was brought to public attention by Rachel Carson's book Silent Spring. One side-effect of DDT is to reduce the thickness of shells on the eggs of predatory birds. The shells sometimes become too thin to be viable, reducing bird populations. This occurs with DDT and related compounds due to the process of bioaccumulation, wherein the chemical, due to its stability and fat solubility, accumulates in organisms' fatty tissues. Also, DDT may biomagnify, which causes progressively higher concentrations in the body fat of animals farther up the food chain. The near-worldwide ban on agricultural use of DDT and related chemicals has allowed some of these birds, such as the peregrine falcon, to recover in recent years. A number of organochlorine pesticides have been banned from most uses worldwide. Globally they are controlled via the Stockholm Convention on persistent organic pollutants. These include: aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, mirex and toxaphene.[citation needed]
Runoff and Percolation
Solid bait and liquid insecticides, especially if improperly applied in a location, get moved by water flow. Often, this happens through nonpoint sources where runoff carries insecticides in to larger bodies of water. As snow melts and rainfall moves over and through the ground, the water picks applied insecticides and deposits them in to larger bodies of water, rivers, wetlands, underground sources of previously potable water, and percolates in to watersheds.[43] This runoff and percolation of insecticides can effect the quality of water sources, harming the natural ecology and thus, indirectly effect human populations through biomagnification and bioaccumulation.
Pollinator decline
Insecticides can kill bees and may be a cause of pollinator decline, the loss of bees that pollinate plants, and colony collapse disorder (CCD),[44] in which worker bees from a beehive or Western honey bee colony abruptly disappear. Loss of pollinators means a reduction in crop yields.[44] Sublethal doses of insecticides (i.e. imidacloprid and other neonicotinoids) affect bee foraging behavior.[45] However, research into the causes of CCD was inconclusive as of June 2007.[46]
Bird decline
Besides the effects of direct consumption of insecticides, populations of insectivorous birds decline due to the collapse of their prey populations. Spraying of especially wheat and corn in Europe is believed to have caused an 80 per cent decline in flying insects, which in turn has reduced local bird populations by one to two thirds.[47]
Alternatives
Instead of using chemical insecticides to avoid crop damage caused by insects, there are many alternative options available now that can protect farmers from major economic losses.[48] Some of them are:
- Breeding crops resistant, or at least less susceptible, to pest attacks.[49]
- Releasing predators, parasitoids, or pathogens to control pest populations as a form of biological control.[50]
- Chemical control like releasing pheromones into the field to confuse the insects into not being able to find mates and reproduce.[51]
- Integrated Pest Management: using multiple techniques in tandem to achieve optimal results.[52]
- Push-pull technique: intercropping with a "push" crop that repels the pest, and planting a "pull" crop on the boundary that attracts and traps it.[53]
Examples
Source:[54]
See also
- Fogger
- Index of pesticide articles
- Insecticide Resistance Action Committee
- Integrated pest management
- Pesticide application
- Sterile insect technique
References
- ↑ IUPAC (2006). "Glossary of Terms Relating to Pesticides". IUPAC. p. 2123. http://www.iupac.org/publications/pac/2006/pdf/7811x2075.pdf.
- ↑ van Emden, H.F.; Peakall, David B. (30 June 1996). Beyond Silent Spring. Springer. ISBN 978-0-412-72800-6. https://books.google.com/books?id=PyjFtiNFVG0C.
- ↑ Delso, N. Simon (2015). "Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites". Environmental Science and Pollution Research 22 (1): 5–34. doi:10.1007/s11356-014-3470-y. PMID 25233913. Bibcode: 2015ESPR...22....5S.
- ↑ "Non-Repellent insecticides". http://store.doyourownpestcontrol.com/crawling-insects/ant-control-products/non-repellent-ant-spray#.
- ↑ "EPA's Regulation of Bacillus thuringiensis (Bt) Crops - Pesticides". 2006-06-28. http://archive.epa.gov/pesticides/biopesticides/web/html/regofbtcrops.html.
- ↑ "dropdata.org". dropdata.org. http://www.dropdata.org.[better source needed]
- ↑ Karl Grandin, ed (1948). "Paul Müller Biography". Les Prix Nobel. The Nobel Foundation. http://nobelprize.org/nobel_prizes/medicine/laureates/1948/muller-bio.html.
- ↑ Vijverberg et al. (1982). "Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves". Nature 295 (5850): 601–603. doi:10.1038/295601a0. PMID 6276777. Bibcode: 1982Natur.295..601V.
- ↑ "Acetylcholinesterase inhibitors: pharmacology and toxicology". Current Neuropharmacology 11 (3): 315–35. May 2013. doi:10.2174/1570159X11311030006. PMID 24179466.
- ↑ 10.0 10.1 10.2 Palmer, WE, Bromley, PT, and Brandenburg, RL. Wildlife & pesticides - Peanuts. North Carolina Cooperative Extension Service. Retrieved on 14 October 2007.
- ↑ "Infographic: Pesticide Planet". Science 341 (6147): 730–731. 2013. doi:10.1126/science.341.6147.730. PMID 23950524. Bibcode: 2013Sci...341..730..
- ↑ "Public Health Statement for DDT, DDE, and DDD". ATSDR. Sep 2002. https://www.atsdr.cdc.gov/ToxProfiles/tp35-c1-b.pdf.
- ↑ "Medical Management Guidelines (MMGs): Chlordane". ATSDR. Apr 18, 2012. https://www.atsdr.cdc.gov/MMG/MMG.asp?id=349&tid=62.
- ↑ "Toxicological Profile for Toxaphene". ATSDR. Aug 1996. pp. 5. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/toxaphene_508.pdf.
- ↑ Class, Thomas J.; Kintrup, J. (1991). "Pyrethroids as household insecticides: analysis, indoor exposure and persistence". Fresenius' Journal of Analytical Chemistry 340 (7): 446–453. doi:10.1007/BF00322420.
- ↑ Soderlund, David (2010). "Chapter 77 – Toxicology and Mode of Action of Pyrethroid Insecticides". in Kreiger, Robert. Hayes' Handbook of Pesticide Toxicology (3rd ed.). Academic Press. pp. 1665–1686. ISBN 978-0-12-374367-1. OCLC 918401061.
- ↑ Fishel, Frederick M. (9 March 2016). "Pesticide Toxicity Profile: Neonicotinoid Pesticides". http://edis.ifas.ufl.edu/pi117.
- ↑ Yamamoto, Izuru (1999). "Nicotine to Nicotinoids: 1962 to 1997". in Yamamoto, Izuru; Casida, John. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 3–27. ISBN 978-4-431-70213-9. OCLC 468555571.
- ↑ Cressey, D (2013). "Europe debates risk to bees". Nature 496 (7446): 408. doi:10.1038/496408a. ISSN 1476-4687. PMID 23619669. Bibcode: 2013Natur.496..408C.
- ↑ Gill, RJ; Ramos-Rodriguez, O; Raine, NE (2012). "Combined pesticide exposure severely affects individual- and colony-level traits in bees". Nature 491 (7422): 105–108. doi:10.1038/nature11585. ISSN 1476-4687. PMID 23086150. Bibcode: 2012Natur.491..105G.
- ↑ "Bees, lies and evidence-based policy". Nature 494 (7437): 283. 2013. doi:10.1038/494283a. ISSN 1476-4687. PMID 23426287. Bibcode: 2013Natur.494..283D.
- ↑ Stoddart, C (2012). "The buzz about pesticides". Nature. doi:10.1038/nature.2012.11626. ISSN 1476-4687.
- ↑ "Ecology: Bumblebees and pesticides". Nature 491 (7422): 43–45. 2012. doi:10.1038/nature11637. ISSN 1476-4687. PMID 23086148. Bibcode: 2012Natur.491...43O.
- ↑ Cressey, D (2013). "Reports spark row over bee-bothering insecticides". Nature. doi:10.1038/nature.2013.12234. ISSN 1476-4687.
- ↑ "Bees & Pesticides: Commission goes ahead with plan to better protect bees". 30 May 2013. http://ec.europa.eu/food/animal/liveanimals/bees/neonicotinoids_en.htm.
- ↑ "Insecticides taking toll on honeybees". Archived from the original on March 18, 2012. https://web.archive.org/web/20120318005423/http://www.columbiatribune.com/news/2012/feb/19/insecticides-taking-toll-on-honeybees/.
- ↑ Yao, Cheng; Shi, Zhao-Peng; Jiang, Li-Ben; Ge, Lin-Quan; Wu, Jin-Cai; Jahn, Gary C. (20 January 2012). "Possible connection between imidacloprid-induced changes in rice gene transcription profiles and susceptibility to the brown plant hopper Nilaparvata lugens Stål (Hemiptera: Delphacidae)". Pesticide Biochemistry and Physiology 102 (3): 213–219. doi:10.1016/j.pestbp.2012.01.003. ISSN 0048-3575. PMID 22544984. PMC 3334832. http://scienceindex.com/stories/2068471/Possible_connection_between_imidaclopridinduced_changes_in_rice_gene_transcription_profiles_and_susceptibility_to_the_brown_plant_hopperNilaparvatalugensStl_Hemiptera_Delphacidae.html.
- ↑ "Fipronil- A Phenylpyrazole Pesticides". https://u.osu.edu/pesticide/2019/05/26/fipronil-a-phenylpyrazole-pesticides/.
- ↑ Nauen, Ralf; Jeschke, Peter; Velten, Robert; Beck, Michael E; Ebbinghaus-Kintscher, Ulrich; Thielert, Wolfgang; Wölfel, Katharina; Haas, Matthias et al. (June 2015). "Flupyradifurone: a brief profile of a new butenolide insecticide" (in en). Pest Management Science 71 (6): 850–862. doi:10.1002/ps.3932. PMID 25351824.
- ↑ "Pesticide Marketed as Safe for Bees Harms Them in Study" (in en). https://www.the-scientist.com/news-opinion/pesticide-marketed-as-safe-for-bees-harms-them-in-study-65734.
- ↑ Tosi, S.; Nieh, J. C. (2019-04-10). "Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto®), on honeybees". Proceedings of the Royal Society B: Biological Sciences 286 (1900): 20190433. doi:10.1098/rspb.2019.0433. PMID 30966981.
- ↑ Tong, Linda; Nieh, James C.; Tosi, Simone (2019-12-01). "Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation" (in en). Chemosphere 237: 124408. doi:10.1016/j.chemosphere.2019.124408. ISSN 0045-6535. PMID 31356997. Bibcode: 2019Chmsp.237l4408T.
- ↑ "Pesticide Fact Sheet- chlorantraniliprole". epa.gov. http://www.epa.gov/opprd001/factsheets/chloran.pdf.
- ↑ "Insect Growth Regulators". http://jenny.tfrec.wsu.edu/opm/displaySpecies.php?pn=-60.
- ↑ Mansour, Ramzi; Grissa-Lebdi, Kaouthar; Suma, Pompeo; Mazzeo, Gaetana; Russo, Agatino (2017-01-05). "Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: host plants, bio-ecological characteristics, natural enemies and pest management strategies – a review". Plant Protection Science (Czech Academy of Agricultural Sciences) 53 (1): 1–14. doi:10.17221/53/2016-pps. ISSN 1212-2580.
- ↑ Isman Murray B (2006). "Botanical Insecticides, Deterrents, And Repellents In Modern Agriculture And An Increasingly Regulated World". Annual Review of Entomology 51: 45–66. doi:10.1146/annurev.ento.51.110104.151146. PMID 16332203.
- ↑ Trapp, S.; Croteau, R. (2001). "Defensive Biosynthesis of Resin in Conifers". Annual Review of Plant Physiology and Plant Molecular Biology 52 (1): 689–724. doi:10.1146/annurev.arplant.52.1.689. PMID 11337413.
- ↑ 38.0 38.1 38.2 Kupferschmidt, K. (2013). "A Lethal Dose of RNA". Science 341 (6147): 732–3. doi:10.1126/science.341.6147.732. PMID 23950525. Bibcode: 2013Sci...341..732K.
- ↑ Zhu, Kun Yan; Palli, Subba Reddy (2020-01-07). "Mechanisms, Applications, and Challenges of Insect RNA Interference". Annual Review of Entomology (Annual Reviews) 65 (1): 293–311. doi:10.1146/annurev-ento-011019-025224. ISSN 0066-4170. PMID 31610134.
- ↑ King, Glenn F.; Hardy, Margaret C. (2013-01-07). "Spider-Venom Peptides: Structure, Pharmacology, and Potential for Control of Insect Pests". Annual Review of Entomology (Annual Reviews) 58 (1): 475–496. doi:10.1146/annurev-ento-120811-153650. ISSN 0066-4170. PMID 23020618.
- ↑ Cole Rosemary A (1976). "Isothiocyanates, nitriles and thiocyanates as products of autolysis of glucosinolates in Cruciferae". Phytochemistry 15 (5): 759–762. doi:10.1016/S0031-9422(00)94437-6. Bibcode: 1976PChem..15..759C.
- ↑ Metcalf, Robert L. (2002). "Insect Control". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a14_263. ISBN 978-3527306732.
- ↑ Environmental Protection Agency (2005). "Protecting Water Quality from Agricultural Runoff". https://www.epa.gov/sites/production/files/2015-09/documents/ag_runoff_fact_sheet.pdf.
- ↑ 44.0 44.1 Wells M (March 11, 2007). "Vanishing bees threaten US crops". www.bbc.co.uk (BBC News). http://news.bbc.co.uk/2/hi/americas/6438373.stm.
- ↑ Colin, M. E.; Bonmatin, J. M.; Moineau, I. et al. (2004). "A method to quantify and analyze the foraging activity of honey bees: Relevance to the sublethal effects induced by systemic insecticides". Archives of Environmental Contamination and Toxicology 47 (3): 387–395. doi:10.1007/s00244-004-3052-y. PMID 15386133.
- ↑ Oldroyd, B.P. (2007). "What's Killing American Honey Bees?". PLOS Biology 5 (6): e168. doi:10.1371/journal.pbio.0050168. PMID 17564497.
- ↑ "Catastrophic collapse in farmland bird populations across France". BirdGuides. 21 March 2018. https://www.birdguides.com/news/catastrophic-collapse-in-farmland-bird-populations-across-france/.
- ↑ Aidley, David (Summer 1976). "Alternatives to insecticides". Science Progress 63 (250): 293–303. PMID 1064167.
- ↑ Russell, GE (1978). Plant Breeding for Pest and Disease Resistance. Elsevier. ISBN 978-0-408-10613-9.
- ↑ "Biological Control and Natural Enemies of Invertebrates Management Guidelines--UC IPM". http://ipm.ucanr.edu/PMG/PESTNOTES/pn74140.html.
- ↑ "Mating Disruption". http://jenny.tfrec.wsu.edu/opm/displaySpecies.php?pn=-80.
- ↑ "Defining IPM | New York State Integrated Pest Management". https://nysipm.cornell.edu/about/defining-ipm/.
- ↑ Cook, Samantha M.; Khan, Zeyaur R.; Pickett, John A. (2007). "The use of push-pull strategies in integrated pest management". Annual Review of Entomology 52: 375–400. doi:10.1146/annurev.ento.52.110405.091407. ISSN 0066-4170. PMID 16968206.
- ↑ "Interactive MoA Classification". 2020-09-16. http://irac-online.org/modes-of-action/.
- ↑ 55.0 55.1 55.2 55.3 "Cinnamon Oil Kills Mosquitoes". www.sciencedaily.com. https://www.sciencedaily.com/releases/2004/07/040716081706.htm.
- ↑ "Cornelia Dick-Pfaff: Wohlriechender Mückentod, 19.07.2004". http://www.wissenschaft.de/wissen/news/243037.html.
- ↑ Comprehensive natural products chemistry (1st ed.). Amsterdam: Elsevier. 1999. p. 306. ISBN 978-0-08-091283-7.
- ↑ Bentley, Ronald (2008). "A fresh look at natural tropolonoids". Nat. Prod. Rep. 25 (1): 118–138. doi:10.1039/B711474E. PMID 18250899.
- ↑ "R.E.D. FACTS: Limonene". EPA – United States Environmental Protection Agency. https://archive.epa.gov/pesticides/reregistration/web/pdf/3083fact.pdf.
- ↑ "BIOPESTICIDES REGISTRATION ACTION DOCUMENT". U.S. Environmental Protection Agency. https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-128838_10-Jun-08.pdf.
- ↑ US EPA, OCSPP (10 August 2020). "Nootkatone Now Registered by EPA" (in en). https://www.epa.gov/pesticides/nootkatone-now-registered-epa.
- ↑ "Oregano Oil Works As Well As Synthetic Insecticides To Tackle Common Beetle Pest". www.sciencedaily.com. https://www.sciencedaily.com/releases/2008/05/080522072339.htm.
- ↑ "Almond farmers seek healthy bees". BBC News. 2006-03-08. http://news.bbc.co.uk/2/hi/science/nature/4780034.stm.
- ↑ 64.0 64.1 Bacteria cornell.edu [|permanent dead link|dead link}}]
Further reading
- McWilliams James E (2008). "'The Horizon Opened Up Very Greatly': Leland O. Howard and the Transition to Chemical Insecticides in the United States, 1894–1927". Agricultural History 82 (4): 468–95. doi:10.3098/ah.2008.82.4.468. PMID 19266680.
External links
| Wikisource has the text of the 1920 Encyclopedia Americana article Insecticide. |
- InsectBuzz.com - Daily updated news on insects and their relatives, including information on insecticides and their alternatives
- International Pesticide Application Research Centre (IPARC)
- Pestworld.org – Official site of the National Pest Management Association
- Streaming online video about efforts to reduce insecticide use in rice in Bangladesh. on Windows Media Player, on RealPlayer
- How Insecticides Work – Has a thorough explanation on how insecticides work.
- University of California Integrated pest management program
- Using Insecticides, Michigan State University Extension
- Example of Insecticide application in the Tsubo-en Zen garden (Japanese dry rock garden) in Lelystad, The Netherlands.
- "IRAC". 2021-03-01. http://irac-online.org/.
 |

