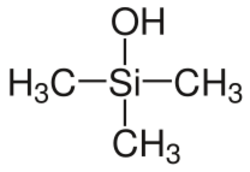
Chemistry:Organosilanols
In organosilicon chemistry, organosilanols are a group of chemical compounds derived from silicon. More specifically, they are carbosilanes derived with a hydroxy group (–OH) on the silicon atom.[1] Organosilanols are the silicon analogs to alcohols. Silanols are more acidic and more basic than their alcohol counterparts and therefore show a rich structural chemistry characterized by hydrogen bonding networks which are particularly well studied for silanetriols.[2][3]
Preparation
Organosilanols can be obtained by hydrolysis of organohalosilanes, such as chlorotrimethylsilane. They can also be prepared by the oxidation of organosilanes with oxidizing agents (R = organic residue):
- [math]\ce{ R2SiCl2{} + 2 HgO ->[-80^{\circ}C][toluene] R2Si(OH)2{} + 2 Hg^0 }[/math]
or by hydrolysis in the alkaline:[4]
- [math]\ce{ (H3C)3SiCl + H2O -> (H3C)3SiOH + HCl }[/math]
- [math]\ce{ R3SiH + H2O -> R3SiOH + H2 }[/math]
The hydrolysis of silyl ethers generally proceeds only slowly:[4]
- [math]\ce{ (H5C2)3SiOC2H5 + H2O -> (H5C2)3SiOH + HOC2H5 }[/math]
Hydrolysis of organosilanes is a first-order reaction. The hydrolysis rate of the Si-H bond depends on the type and number of organic residues. Thus, the hydrolysis rate of trialkylsilanes is significantly slower than that of triarylsilanes. This can be explained by a stronger increase in electron density on the silicon atom by the alkyl groups. Correspondingly, the reaction rate of the tri-n-alkylsilanes decreases in the series of ethyl, propyl, butyl groups. Trialkylsilanes with n-alkyl residues react by a factor of 10 faster than the analogous silanes with branched alkyl residues.[5]
Classification
Depending on the substitution pattern of the silicon atom, a further distinction can be made. Organosilanols are classified as:
- organosilanetriols, when three hydroxy groups and an organic residue are bound to a silicon atom, e. g. methylsilanetriol, phenylsilanetriol
- organosilandiols, when two hydroxy groups and two organic residues are bound to a silicon atom, e. g. dimethylsilanediol, diphenylsilanediol
- organosilanols, when one hydroxy group and three organic residues are bound to a silicon atom, e. g. trimethylsilanol, triethylsilanol or triphenylsilanol.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "silanols". doi:10.1351/goldbook.S05664
- ↑ Paul D. Lickiss (1995), "The synthesis and structure of organosilanols" (in en), Advances in Inorganic Chemistry 42: 147–262, doi:10.1016/S0898-8838(08)60053-7
- ↑ Rudolf Pietschnig, Stefan Spirk (2016), "The Chemistry of Organo Silanetriols" (in en), Coordination Chemistry Reviews 323: 87–106, doi:10.1016/j.ccr.2016.03.010
- ↑ 4.0 4.1 Barry Arkles. "Silanes" (pdf). pp. 50. http://www.gelest.com/wp-content/uploads/Silicon_Hydrides.pdf.
- ↑ G. Schott, C. Harzdorf (October 1960), "Silane. I Alkalische Solvolyse von Triorganosilanen" (in de), Zeitschrift für anorganische und allgemeine Chemie 306 (3–4): 180–190, doi:10.1002/zaac.19603060306
 |