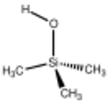
Chemistry:Trimethylsilanol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trimethylsilanol[1] | |||
| Other names
Hydroxy(trimethyl)silane[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
| MeSH | Trimethylsilanol | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H10OSi | |||
| Molar mass | 90.197 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Boiling point | 99 °C (210 °F; 372 K) | ||
| Vapor pressure | 21 mbar (20 °C) [2] | ||
| Related compounds | |||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethylsilanol (TMS) is an organosilicon compound with the formula (CH3)3SiOH. The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.[3][4]
Occurrence
TMS is a contaminant in the atmospheres of spacecraft, where it arises from the degradation of silicone-based materials.[5] Specifically, it is the volatile product from the hydrolysis of polydimethylsiloxane, which are generally terminated with trimethylsilyl groups:
- (CH3)3SiO[Si(CH3)2O]nR + H2O → (CH3)3SiOH + HO[Si(CH3)2O]nR
TMS and related volatile siloxanes are formed by hydrolysis of silicones-based containing materials, which are found in detergents and cosmetic products.
Traces of trimethylsilanol, together with other volatile siloxanes, are present in biogas and landfill gas, again resulting from the degradation of silicones. As their combustion forms particles of silicates and microcrystalline quartz, which cause abrasion of combustion engine parts, they pose problems for the use of such gases in combustion engines.[6]
Production
Trimethylsilanol cannot be produced by simple hydrolysis of chlorotrimethylsilane as this reaction leads to the etherification product hexamethyldisiloxane, because of the by-product hydrochloric acid.[7]Failed to parse (syntax error): {\displaystyle \ce{2ClSi(CH3)3 ->[{}\atop\ce{+H2O, -HCl}]} \ce{2HOSi(CH3)3 ->[{}\atop\ce{-H2O}] (CH3)3Si-O-Si(CH3)3}}
Trimethylsilanol is accessible by weakly basic hydrolysis of chlorotrimethylsilane, since the dimerization can thus be avoided.[8] Trimethylsilanol can also be obtained by the basic hydrolysis of hexamethyldisiloxane.[9]
Reactions
Trimethylsilanol is a weak acid with a pKa value of 11.[10] The acidity is comparable to that of orthosilicic acid, but much higher than the one of alcohols like tert-butanol (pKa 19[10]). Deprotonation with sodium hydroxide gives sodium trimethylsiloxide.
TMS reacts with the silanol groups (R3SiOH) giving silyl ethers.
Structure
In terms of its structure, the molecule is tetrahedral. The compound forms monoclinic crystals.[11]
Additional properties
The heat of evaporation is 45.64 kJ·mol−1, the evaporation entropy 123 J·K−1·mol−1.[2] The vapor pressure function according to Antoine is obtained as log10(P/1 bar) = A − B/(T + C) (P in bar, T in K) with A = 5.44591, B = 1767.766 K and C = −44.888 K in a temperature range from 291 K to 358 K.[2] Below the melting point at −4.5 °C,[12] The 1H NMR in CDCl3 shows a singlet at δ=0.14 ppm.[13]
Bioactivity
Like other silanols, trimethylsilanol exhibits antimicrobial properties.[14]
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 696. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 Grubb, W.T.; Osthoff, R.C.: Physical Properties of Organosilicon Compounds. II. Trimethylsilanol and Triethylsilanol in J. Am. Chem. Soc. 75 (1953) 2230–2232; doi:10.1021/ja01105a061.
- ↑ Paul D. Lickiss: The Synthesis and Structure of Organosilanols, Advances in Inorganic Chemistry 1995, Volume 42, Pages 147–262, doi:10.1016/S0898-8838(08)60053-7.
- ↑ Vadapalli Chandrasekhar, Ramamoorthy Boomishankar, Selvarajan Nagendran: Recent Developments in the Synthesis and Structure of Organosilanols, Chem. Rev. 2004, volume 104, pp 5847–5910, doi:10.1021/cr0306135.
- ↑ Trimethylsilanol, Harold L. Kaplan, Martin E. Coleman, John T. James: Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants, Volume 1 (1994).
- ↑ http://epics.ecn.purdue.edu/bgi/Documents/Fall%25202009/Removal_of_Siloxanes_.pdf [|permanent dead link|dead link}}]
- ↑ Didier Astruc: Organometallic Chemistry and Catalysis. Springer Science & Business Media, 2007, ISBN 978-3-540-46129-6, S. 331 ([1], p. 331, at Google Books).
- ↑ J.A. Cella, J.C. Carpenter: Procedures for the preparation of silanols in J. Organomet. Chem. 480 (1994), 23–23; doi:10.1016/0022-328X(94)87098-5
- ↑ M. Lovric, I. Cepanec, M. Litvic, A. Bartolincic, V. Vinkovic: Croatia Chem. Acta 80 (2007), 109–115
- ↑ 10.0 10.1 T. Kagiya, Y. Sumida, T. Tachi: An Infrared Spectroscopic Study of hydrogen Bonding Interaction. Structural Studies of Proton-donating and -accepting Powers in Bull. Chem. Soc. Jpn. 43 (1970), 3716–3722.
- ↑ R. Minkwitz, S. Schneider: Die Tieftemperaturkristallstruktur von Trimethylsilanol. In: Zeitschrift für Naturforschung B. 53, 1998, S. 426–429 (PDF, freier Volltext).
- ↑ Batuew et al. in Doklady Akademii Nauk SSSR 95 (1954) 531.
- ↑ Hamada, Tomoaki; Manabe, Kai; Kobayashi, Shū (13 January 2006). "Catalytic Asymmetric Mannich-Type Reactions Activated by ZnF2 Chiral Diamine in Aqueous Media". Chemistry - A European Journal 12 (4): 1205–1215. doi:10.1002/chem.200500673. PMID 16267871. https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.200500673. Retrieved 16 March 2021.
- ↑ Yun-mi Kim, Samuel Farrah, Ronald H. Baney (2006). "Silanol – A novel class of antimicrobial agent". Electronic Journal of Biotechnology 9 (2): 176–180. doi:10.2225/vol9-issue2-fulltext-4. http://www.ejbiotechnology.info/content/vol9/issue2/full/4/index.html.
 |