Chemistry:Oxamate
| |
| Names | |
|---|---|
| Other names
Carbamoylformate; amino(oxo)acetate; 2-oxo-2-aminoacetate
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| C2H2NO3− | |
| Molar mass | 88.043 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
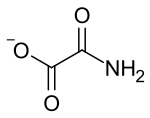
Oxamate is the carboxylate anion of oxamic acid.[1] Oxamate has a molecular formula of C2H2NO3− and is an isosteric form of pyruvate. Salts and esters of oxamic acid are known collectively as oxamates.
Oxamate is a competitive inhibitor of the enzyme lactate dehydrogenase.[2] Oxamate is a possible pyruvate analog that has the ability to halt lactate production by inhibiting lactate dehydrogenase, effectively stopping the conversation process of pyruvate to lactate.[3]
Oxamate, as a lactate dehydrogenase (LDH) inhibitor, plus phenformin, an anti-diabetic agent, has been tested in conjunction with one another and it was shown that this combination has potential anti-cancer properties.[4] Phenformin when administered by itself has a high incidence of lactic acidosis. Due to the inherent ability of oxamate to prevent the conversion of pyruvate to lactate, oxamate can be used to counterbalance the side effects of phenformin.[4]
Oxamate also plays inhibiting roles with oxaloacetate, an important intermediate for the citric acid cycle. Oxamate competes and binds to the carboxyl transferase domain active site, and reverses the reaction of oxalaoacetate decarboxylation by pyruvate carboxylase.[5]
References
- ↑ Watts, Henry (1866). A Dictionary of Chemistry. Longman, Green, Roberts & Green. pp. 279–. https://books.google.com/books?id=r4zPAAAAMAAJ&pg=PA279.
- ↑ Zhai, Xiaoming; Yang, Yang; Wan, Jianmei; Zhu, Ran; Wu, Yiwei (2013-09-19). "Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells". Oncology Reports 30 (6): 2983–2991. doi:10.3892/or.2013.2735. PMID 24064966.
- ↑ "Oxamate Improves Glycemic Control and Insulin Sensitivity via Inhibition of Tissue Lactate Production in db/db Mice". PLOS ONE 11 (3): e0150303. 2016-03-03. doi:10.1371/journal.pone.0150303. PMID 26938239.
- ↑ 4.0 4.1 "Synergistic anti-cancer effect of phenformin and oxamate". PLOS ONE 9 (1): e85576. 2014. doi:10.1371/journal.pone.0085576. PMID 24465604.
- ↑ "Oxamate is an alternative substrate for pyruvate carboxylase from Rhizobium etli" (in EN). Biochemistry 52 (17): 2888–94. April 2013. doi:10.1021/bi400075t. PMID 23560609.
 |
