Chemistry:Oximide
| |
| Names | |
|---|---|
| IUPAC name
Aziridine-2,3-dione
| |
| Other names
Oxalimide; 2,3-Aziridinedione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C2HNO2 | |
| Molar mass | 71.035 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
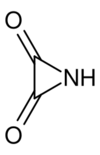
Oximide is an unstable chemical compound, the cyclic imide of oxalic acid. Other names for this are the systematic name 2,3-Aziridinedione or oxalimide. The chemical formula is C2HNO2. Its core is a three member heterocycle, aziridine.
Production
In 1886 Ost and Mente claimed to produce oximide by the reaction of oxamic acid with phosphorus pentachloride (PCl5).[1] However, a product with a six member ring, tetraketopiperazine, may have been produced instead. Later attempts to reproduce the production of oximide by this method were a failure.[2] The first successful manufacture of oximide was by Hiromu Aoyama, Masami Sakamoto, and Yoshimori Omote in 1980.[3]
Properties
Aziridine-2,3-dione has an infrared absorption band at 1954 cm−1.[3]
Related
The term "oximide" has also been used for oximes.[4]
Derivatives of oximide exist where the hydrogen atom is substituted by other organic groups such as methyl or phenyl. When 4-methyl-1,2,4-triazolinedione is irradiated by ultraviolet light with wavelength 335 nm in a noble gas matrix, some methylaziridine-2,3-dione is made (along with isocyanates, carbon monoxide and dinitrogen). Similarly 4-phenyl-1,2,4-triazolinedione irradiated by ultraviolet light with wavelength 310 nm makes some phenylaziridine-2,3-dione. Shorter wavelength ultraviolet light decomposes these compounds to isocyanates (-NCO).[5]
Another method to produce oximide derivatives is by the photolysis of substituted diphenylmaleylimide ozonide at liquid nitrogen temperature (77K) in a potassium bromide matrix. Derivatives made this way are methyl, isopropyl and phenylethyl-aziridine-2,3-dione. These compounds are unstable at higher temperatures, and when heated, decompose to carbon monoxide and isocyanates.[3]
References
- ↑ Sudborough, J. J.; Bernthsen, A. (1922). A Textbook of Organic Chemistry. London: Blackie and Sons. p. 245. https://archive.org/details/in.ernet.dli.2015.513637.
- ↑ de Mouilpied, Alfred Theophilus; Rule, Alexander (1907). "XVI.—Tetraketopiperazine". J. Chem. Soc., Trans. 91: 176–183. doi:10.1039/CT9079100176. https://zenodo.org/record/1770094.
- ↑ 3.0 3.1 3.2 Aoyama, Hiromu; Sakamoto, Masami; Omote, Yoshimori (September 1980). "Aziridine-2,3-diones". Journal of the American Chemical Society 102 (22): 6902–6903. doi:10.1021/ja00542a065.
- ↑ Pomfret, H. W.; Roberts, William (January 1895). "V. Organic oximides.—A research on their pharmacology". Philosophical Transactions of the Royal Society of London B 186: 223–320. doi:10.1098/rstb.1895.0005.
- ↑ Risi, Florence; Pizzala, Louis; Carles, Micheline; Verlaque, Patrick; Aycard, Jean-Pierre (January 1996). "Photolysis of Matrix-Isolated 4-R-1,2,4-triazoline-3,5-diones: Identification of Aziridine-2,3-dione Transients". The Journal of Organic Chemistry 61 (2): 666–670. doi:10.1021/jo950778g. PMID 11666989.
 |
