Chemistry:Palmitoleic acid
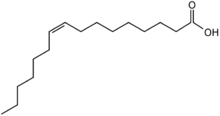
| |
| Names | |
|---|---|
| Preferred IUPAC name
(9Z)-Hexadec-9-enoic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H30O2 | |
| Molar mass | 254.414 g·mol−1 |
| Density | 0.894 g/cm3 |
| Melting point | −0.1 °C (31.8 °F; 273.0 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Palmitoleic acid, or (9Z)-hexadec-9-enoic acid, is an omega-7 monounsaturated fatty acid (16:1n-7) with the formula CH3(CH2)5CH=CH(CH2)7COOH. It is a rare component of fats.[1] It is a common constituent of the glycerides of human adipose tissue.[citation needed] It is present in all tissues but, in general, found in higher concentrations in the liver.
It is biosynthesized from palmitic acid by the action of the enzyme Stearoyl-CoA desaturase-1.
Animal and cell culture studies indicate that palmitoleic acid is anti-inflammatory, and improves insulin sensitivity in liver and skeletal muscles, but more studies are required to establish its actions in humans.[2] Many of the effects of palmitoleic acid are due to its activation of PPAR-alpha.[2]
Dietary sources
Palmitoleic acid is found in trace amounts in most foods except for sardine oil, which contains 15% of this acid as a component of triglycerides.[1]
Other dietary sources of palmitoleic acid include breast milk,[3] a variety of animal fats, vegetable oils, and marine oils. Macadamia oil (Macadamia integrifolia) and sea buckthorn oil (Hippophae rhamnoides) are botanical sources with high concentrations, containing 17% and 19-29% palmitoleic acid, respectively.[citation needed][4][5]
References
- ↑ 1.0 1.1 Anneken, David J.; Both, Sabine; Christoph, Ralf; Fieg, Georg; Steinberner, Udo; Westfechtel, Alfred (2006). "Fatty Acids". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_245.pub2. ISBN 9783527303854.
- ↑ 2.0 2.1 "Is Palmitoleic Acid a Plausible Nonpharmacological Strategy to Prevent or Control Chronic Metabolic and Inflammatory Disorders?". Molecular Nutrition & Food Research 62 (1): 1700504. 2018. doi:10.1002/mnfr.201700504. PMID 28980402. https://eprints.soton.ac.uk/414390/1/Palmitoleic_review_FINAL_Revised3.pdf.
- ↑ "Fatty acid composition of breast milk from Nigerian and Japanese women". J Nutr Sci Vitaminol (Tokyo) 37 (4): 435–42. 1991. doi:10.3177/jnsv.37.435. PMID 1765848.
- ↑ "Nuts, macadamia nuts, raw". NutritionData.com. http://www.nutritiondata.com/facts/nut-and-seed-products/3123/2.
- ↑ Li, Thomas S. C.; Thomas H. J. Beveridge (2003). Sea Buckthorn (Hippophae rhamnoides L.) : Production and Utilization. Ottawa, Ontario: NRC Research Press. pp. 54–55. ISBN 0-660-19007-9. http://www.ovid.com/site/catalog/Book/2738.jsp?top=2&mid=3&bottom=7&subsection=11.
 |

