Chemistry:Patchoulol
Patchoulol or patchouli alcohol (C15H26O) is a sesquiterpene alcohol found in patchouli (Pogostemon cablin).[1] Patchouli oil is an important material in perfumery.[2] The (−)-optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is obtained industrially from patchouli leaf fermentation.[3]
Patchoulol is also used in the synthesis of the chemotherapy drug Taxol.[citation needed]
Structure determination
Gal first isolated patchouli alcohol in 1869, and Montgolfier later formulated its chemical composition (correctly) as C15H26O.[4] Early structural investigation soon established the presence of a saturated tricyclic tertiary alcohol.[5] After several years of careful degradation study, Büchi and co-workers proposed in 1961 that patchouli alcohol had the structure 1. A subsequent synthesis of material which corresponded to an authentic sample of natural patchouli alcohol appeared to verify Büchi's proposal.[6]
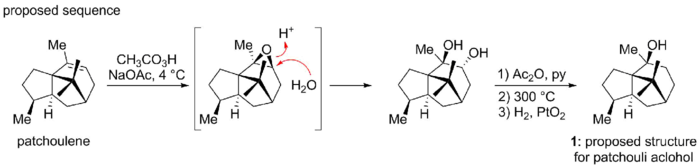
![Actual sequence for the synthesis of patchouli alcohol. Contains embedded bicyclo[2.2.2]octane motif.](/wiki/images/thumb/6/65/Actual_sequence_patchouli_alcohol.png/700px-Actual_sequence_patchouli_alcohol.png)
However, Dunitz and co-workers serendipitously discovered in 1963 that Büchi's structure is in fact incorrect. Dunitz et al. had undertaken X-ray analysis of the patchouli alcohol diester with chromic acid, intending to determine the Cr-O-C angles. In the course of their analysis they could not reconcile the X-ray evidence with the "known" structure 1.[7] In a joint paper with Büchi, they collectively proposed that patchouli alcohol in fact had the novel structure 2. The discrepancy had resulted from an unanticipated skeletal rearrangement when patchoulene was treated with peroxy acid in Büchi's confirmatory synthesis. The rearranged molecule coincidentally exhibited the correct natural product architecture.[8]
See also
References
- ↑ Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. (2006). "The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases". Archives of Biochemistry and Biophysics 454 (2): 123–136. doi:10.1016/j.abb.2006.08.006. PMID 16970904.
- ↑ "Духи с запахом пачулей - Лучшие парфюмы с пачулями - Описания духов с ароматом пачулей - Парфюмерия с пачулями.". https://duhipatchouli.ru/.
- ↑ Sell, Charles S. (2006). "Terpenoids". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.2005181602120504.a01.pub2. ISBN 0471238961.
- ↑ Büchi, G.; Erickson, R. E.; Wakabyashi, N. (1961). "Terpenes. XVI. Constitution of Patchouli Alcohol and Absolute Configuration of Cedrene". Journal of the American Chemical Society 83 (4): 927–938. doi:10.1021/ja01465a042. Bibcode: 1961JAChS..83..927B.
- ↑ Simonsen, J.; Barton, D. H. R. (1952). The Terpenes. 111. Cambridge University Press, London.
- ↑ Büchi, G.; Macleod, W. D. (1962). "Synthesis of Patchouli Alcohol". Journal of the American Chemical Society 84 (16): 3205–3206. doi:10.1021/ja00875a047. Bibcode: 1962JAChS..84.3205B.
- ↑ Dobler, M.; Dunitz, J. D.; Gubler, B.; Weber, H. P.; Büchi, G.; Padilla, O. J. (1963). "The Structure of Patchouli Alcohol". Proc. Chem. Soc. December: 383. doi:10.1039/PS9630000357.
- ↑ Nicolaou, K. C.; Snyder, S. A. (2005). "Chasing Molecules That Were Never There: Misassigned Natural Products and the Role of Chemical Synthesis in Modern Structure Elucidation". Angewandte Chemie International Edition 44 (7): 1012–1044. doi:10.1002/anie.200460864. PMID 15688428. Bibcode: 2005ACIE...44.1012N.
External links
 |
