Chemistry:Chromic acid
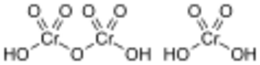 Dichromic acid (left) and chromic acid (right)
| |
| Names | |
|---|---|
| IUPAC names
Chromic acid
Dichromic acid | |
| Systematic IUPAC name
Dihydroxidodioxidochromium | |
| Other names
Chromic(VI) acid
Tetraoxochromic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 25982 | |
PubChem CID
|
|
| UNII | |
| UN number | 1755 1463 |
| |
| |
| Properties | |
| H 2CrO 4 (chromic acid) H 2Cr 2O 7 (dichromic acid) | |
| Molar mass | 118.008 g/mol (chromic acid) 218.001 g/mol (dichromic acid) |
| Appearance | Dark purplish-red sand-like crystalline solid or powder[clarification needed] |
| Odor | Odorless |
| Density | 1.201 g/cm3[clarification needed] |
| Melting point | 197 °C (387 °F; 470 K) [clarification needed] |
| Boiling point | 250 °C (482 °F; 523 K) (decomposes)[clarification needed] |
| 169 g/(100 mL)[clarification needed] | |
| Acidity (pKa) | −0.8 to 1.6 (chromic acid) |
| Conjugate base | Chromate and dichromate |
| Hazards | |
| Main hazards | highly toxic, carcinogen, corrosive |
| GHS pictograms |      
|
| GHS Signal word | Danger |
| H271, H300, H301, H310, H314, H317, H330, H334, H340, H341, H350, H361, H372, H410 | |
| P201, P202, P210, P220, P221, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P283, P284, P285, P301+310, P301+330+331, P302+350, P302+352, P303+361+353, P304+340, P304+341 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
51.9 mg/kg (H 2CrO 4 · 2Na, rat, oral)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.005 mg/m3[1] |
REL (Recommended)
|
TWA 0.001 mg Cr(VI)/m3[1] |
IDLH (Immediate danger)
|
15 mg Cr(VI)/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromic acid is jargon for a solution of formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.[3]
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, H
2CrO
4 of which the trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (and a valence of VI or 6). It is a strong and corrosive oxidising agent and a moderate carcinogen.
Molecular chromic acid

Molecular chromic acid, H
2CrO
4, in principle, resembles sulfuric acid, H
2SO
4. It would ionize accordingly:
- H
2CrO
4 ⇌ [HCrO
4]−
+ H+
The pKa for the equilibrium is not well characterized. Reported values vary between about −0.8 to 1.6.[4] The structure of the mono anion has been determined by X-ray crystallography. In this tetrahedral oxyanion, three Cr-O bond lengths are 156 pm and the Cr-OH bond is 201 pm[5]
[HCrO
4]−
condenses to form dichromate:
- 2 [HCrO
4]−
⇌ [Cr
2O
7]2− + H
2O, logKD = 2.05.
Furthermore, the dichromate can be protonated:
- [HCr
2O
7]−
⇌ [Cr
2O
7]2− + H+
, pKa = 1.8[6]
Loss of the second proton occurs in the pH range 4–8, making the ion [HCrO
4]−
a weak acid.[citation needed]
Molecular chromic acid could in principle be made by adding chromium trioxide to water (cf. manufacture of sulfuric acid).
- CrO
3 + H
2O ⇌ H
2CrO
4
In practice, the reverse reaction occurs: molecular chromic acid dehydrates. Some insights can be gleaned from observations on the reaction of dichromate solutions with sulfuric acid. The first colour change from orange to red signals the conversion of dichromate to chromic acid. Under these conditions deep red crystals of chromium trioxide precipitate from the mixture, without further colour change.
Chromium trioxide is the anhydride of molecular chromic acid. It is a Lewis acid and can react with a Lewis base, such as pyridine in a non-aqueous medium such as dichloromethane (Collins reagent).

2Cr
4O
13 · 2H2O, one component of concentrated "chromic acid". The H-atom positions are calculated, not observed. Color code: red = O, white = H, blue = Cr.[7]
Higher chromic acids with the formula H
2Cr
nO
(3n+1) are probable components of concentrated solutions of chromic acid.
Uses
Chromic acid is an intermediate in chromium plating, and is also used in ceramic glazes, and colored glass. Because a solution of chromic acid in sulfuric acid (also known as a sulfochromic mixture or chromosulfuric acid) is a powerful oxidizing agent, it can be used to clean laboratory glassware, particularly of otherwise insoluble organic residues. This application has declined due to environmental concerns.[8] Furthermore, the acid leaves trace amounts of paramagnetic chromic ions (Cr3+) that can interfere with certain applications, such as NMR spectroscopy. This is especially the case for NMR tubes.[9] Piranha solution can be used for the same task, without leaving metallic residues behind.
Chromic acid was widely used in the musical instrument repair industry, due to its ability to "brighten" raw brass. A chromic acid dip leaves behind a bright yellow patina on the brass. Due to growing health and environmental concerns, many have discontinued use of this chemical in their repair shops.
It was used in hair dye in the 1940s, under the name Melereon.[10]
It is used as a bleach in black and white photographic reversal processing.[11]
Reactions
Chromic acid is capable of oxidizing many kinds of organic compounds and many variations on this reagent have been developed:
- Chromic acid in aqueous sulfuric acid and acetone is known as the Jones reagent, which will oxidize primary and secondary alcohols to carboxylic acids and ketones respectively, while rarely affecting unsaturated bonds.[12]
- Pyridinium chlorochromate is generated from chromium trioxide and pyridinium chloride. This reagent converts primary alcohols to the corresponding aldehydes (R–CHO).[12]
- Collins reagent is an adduct of chromium trioxide and pyridine used for diverse oxidations.
- Chromyl chloride, CrO
2Cl
2 is a well-defined molecular compound that is generated from chromic acid.
Illustrative transformations
- Oxidation of methylbenzenes to benzoic acids.[13]
- Oxidative scission of indene to homophthalic acid.[14]
- Oxidation of secondary alcohol to ketone (cyclooctanone)[15] and nortricyclanone.[16]
Use in qualitative organic analysis
In organic chemistry, dilute solutions of chromic acid can be used to oxidize primary or secondary alcohols to the corresponding aldehydes and ketones. Similarly, it can also be used to oxidize an aldehyde to its corresponding carboxylic acid. Tertiary alcohols and ketones are unaffected. Because the oxidation is signaled by a color change from orange to brownish green (indicating chromium being reduced from oxidation state +6 to +3), chromic acid is commonly used as a lab reagent in high school or undergraduate college chemistry as a qualitative analytical test for the presence of primary or secondary alcohols, or aldehydes.[12]
Alternative reagents
In oxidations of alcohols or aldehydes into carboxylic acids, chromic acid is one of several reagents, including several that are catalytic. For example, nickel(II) salts catalyze oxidations by bleach (hypochlorite).[17] Aldehydes are relatively easily oxidised to carboxylic acids, and mild oxidising agents are sufficient. Silver(I) compounds have been used for this purpose. Each oxidant offers advantages and disadvantages. Instead of using chemical oxidants, electrochemical oxidation is often possible.
Safety
Hexavalent chromium compounds (including chromium trioxide, chromic acids, chromates, chlorochromates) are toxic and carcinogenic. Chromium trioxide and chromic acids are strong oxidisers and may react violently if mixed with easily oxidisable organic substances.
Chromic acid burns are treated with a dilute sodium thiosulfate solution.[18]
Notes
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0138". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0138.html.
- ↑ "Chromic acid and chromates". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/1333820.html.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ IUPAC SC-Database A comprehensive database of published data on equilibrium constants of metal complexes and ligands
- ↑ Mukherjee, A. K.; Mukhopadhaya, A.; Mukherjee, M.; Ray, S. (1994). "Two Mononuclear Tetraphenylphosphonium Oxochromium Complexes: (PPh4)[CrVO3(H2O)] and (PPh4)[CrVIO3(OH)]". Acta Crystallographica Section C Crystal Structure Communications 50 (9): 1401–1404. doi:10.1107/S0108270194003148. Bibcode: 1994AcCrC..50.1401M.
- ↑ Brito, F.; Ascanioa, J.; Mateoa, S.; Hernándeza, C.; Araujoa, L.; Gili, P.; Martín-Zarzab, P.; Domínguez, S. et al. (1997). "Equilibria of Chromate(VI) Species in Acid Medium and ab initio Studies of These Species". Polyhedron 16 (21): 3835–3846. doi:10.1016/S0277-5387(97)00128-9.
- ↑ Kulikov, Vladislav; Meyer, Gerd (2013). "Dihydronium Tetrachromate(VI), (H3O)2Cr4O13". Acta Crystallographica Section e Structure Reports Online 69 (2): i13. doi:10.1107/S1600536813001608. PMID 23424393. Bibcode: 2013AcCrE..69I..13K.
- ↑ J. M. McCormick (2006-06-30). "Cleaning Glassware". Truman State University. http://chemlab.truman.edu/Miscellaneous_files/Cleaning.htm.
- ↑ "NMR-010: Proper Cleaning Procedures for NMR Sample Tubes". Wilmad. http://www.wilmad-labglass.com/services/NMR_010.jsp.
- ↑ "Watson v Buckley, Osborne, Garrett & Co Ltd and Wyrovoys Products Ltd [1940] 1 All ER 174". http://www.uniset.ca/other/cs3/19401AER174.html.
- ↑ "Fomapan R". Foma. http://www.fotofachversand.at/pdf/Foma_Fomapan_R_Datenblatt.pdf.
- ↑ 12.0 12.1 12.2 Freeman, F. "Chromic Acid" Encyclopedia of Reagents for Organic Synthesis (2001) John Wiley & Sons, doi:10.1002/047084289X.rc164
- ↑ Kamm O.; Matthews, A. O. (1941). "p-Nitrobenzoic Acid". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV1P0392.; Collective Volume, 1, pp. 392
- ↑ Grummitt, O.; Egan, R.; Buck, A.. "Homophthalic Acid and Anhydride". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV3P0449.; Collective Volume, 3, pp. 449 (1955
- ↑ Eisenbraun, E. J.. "Cyclooctanone". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV5P0310.; Collective Volume, 5, pp. 310 (1973
- ↑ Meinwald, J.; Crandall, J.; Hymans W. E.. "Nortricyclanone". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv5p0866.; Collective Volume, 5, pp. 866
- ↑ J. M. Grill; J. W. Ogle; S. A. Miller (2006). "An Efficient and Practical System for the Catalytic Oxidation of Alcohols, Aldehydes, and α,β-Unsaturated Carboxylic Acids". J. Org. Chem. 71 (25): 9291–9296. doi:10.1021/jo0612574. PMID 17137354.
- ↑ Hettiaratchy, Shehan; Dziewulski, Peter (2004-06-12). "Pathophysiology and types of burns". BMJ: British Medical Journal 328 (7453): 1427–1429. doi:10.1136/bmj.328.7453.1427. ISSN 0959-8138. PMID 15191982.
References
- Alcohols from Carbonyl Compounds: Oxidation-Reduction and Organometallic Compounds[yes|permanent dead link|dead link}}] (PDF)
External links
- International Chemical Safety Card 1194
- NIOSH Pocket Guide to Chemical Hazards. "#0138". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0138.html.
- IARC Monograph "Chromium and Chromium compounds"
 |

