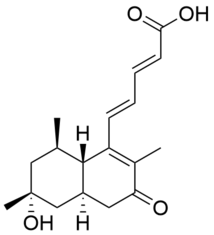
Chemistry:Penitanzacid F
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E,4E)-5-((4aR,6R,8R,8aS)-6-hydroxy-2,6,8-trimethyl-3-oxo-3,4,4a,5,6,7,8,8a-octahydronaphthalen-1-yl)penta-2,4-dienoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C18H24O4 | |
| Molar mass | 304.39 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Penitanzacid F was found as one of the twelve new tanzawaic acid derivatives, which were the secondary metabolites of the fungi Pencillum sp. KWF32 isolated from the tissues of Bathymodiolus sp. collected in the cold spring area of the South China Sea in 2021.[1]
It may have anticoccidial, cytotoxic, lipid-lowering,[2] superoxide anion production inhibiting, bacterial conjugation inhibiting, and NO production inhibiting properties as a tanzawaic acid derivative.[1]
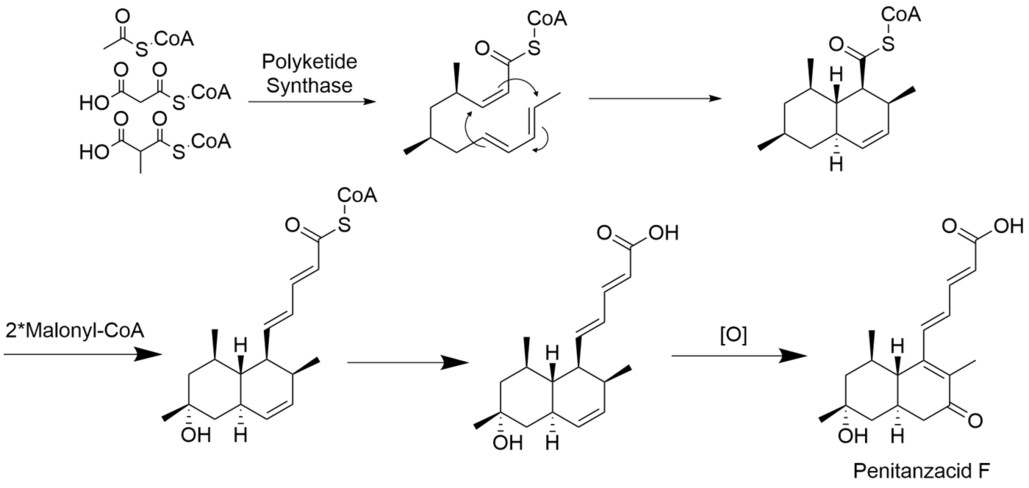
Structure and biosynthesis
The biosynthesis of Penitanzacid F starts from one acetyl-CoA, two methylmalonyl-CoA and three malonyl-CoA molecules with polyketide synthase (PKS). Then the product undergoes Diels-Alder Cyclization, chain elongation with two malonyl-CoA, and is oxidized to penitanzaicacid F.
References
- ↑ Jump up to: 1.0 1.1 Wang, Jiaqi; Li, Taiwei; Wang, Pinmei; Ding, Wanjing; Xu, Jinzhong (2022-05-27). "Tanzawaic Acids from a Deep-Sea Derived Penicillium Species" (in en). Journal of Natural Products 85 (5): 1218–1228. doi:10.1021/acs.jnatprod.1c01020. ISSN 0163-3864. PMID 35420798. https://pubs.acs.org/doi/10.1021/acs.jnatprod.1c01020.
- ↑ Yu, Guihong; Wang, Shuai; Wang, Lu; Che, Qian; Zhu, Tianjiao; Zhang, Guojian; Gu, Qianqun; Guo, Peng et al. (2018-01-12). "Lipid-Lowering Polyketides from the Fungus Penicillium Steckii HDN13-279" (in en). Marine Drugs 16 (1): 25. doi:10.3390/md16010025. ISSN 1660-3397. PMID 29329204.
 |

