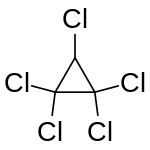
Chemistry:Pentachlorocyclopropane
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
1,1,2,2,3-Pentachlorocyclopropane
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| C3HCl5 | |
| Molar mass | 214.29 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Mild, minty[1] |
| Density | 1.668 g/cm3[2] |
| Boiling point | 56 °C[1] |
Refractive index (nD)
|
1.51[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pentachlorocyclopropane is a chlorinated cyclopropane with the chemical formula C
3HCl
5. It is a colourless liquid with a faint minty odour.[1] It is thermally unstable above 100 °C; decomposition gives 1,1,3,3,3-pentachloropropene by isomerisation.[3] Pentachlorocyclopropane can be obtained by the addition of dichlorocarbene into trichloroethylene in presence of a base.[4] Pentachlorocyclopropane itself gives tetrachlorocyclopropene when reacted with a base such as potassium hydroxide by means of dehydrohalogenation.[5]
References
- ↑ Jump up to: 1.0 1.1 1.2 Pentachlorocyclopropane Stephen W. Tobey and Robert West The University of Wisconsin (1965)
- ↑ Jump up to: 2.0 2.1 Yaws, C. L. (2015). The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics.
- ↑ Organic Reaction Mechanisms 1966: An Annual Survey Covering the Literature Dated December 1965 Through November 1966. Page 228
- ↑ Strain and Its Implications in Organic Chemistry: Organic Stress and Reactivity. (2012). Page 384
- ↑ Advances in Alicyclic Chemistry. (2013). Elsevier Science. page 57
 |

