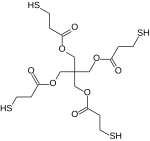
Chemistry:Pentaerythritol tetrakis(3-mercaptopropionate)
From HandWiki
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
[3-(3-sulfanylpropanoyloxy)-2,2-bis(3-sulfanylpropanoyloxymethyl)propyl] 3-sulfanylpropanoate
| |
| Other names
Pentaerythritol tetrakis(3-mercaptopropionate)
Pentaerythritol tetra(3-mercaptopropionate) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H28O8S4 | |
| Molar mass | 488.64 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 600.431 °C (1,112.776 °F; 873.581 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pentaerythritol tetrakis(3-mercaptopropionate) is an organic compound which is derived from pentaerythritol fully esterified with four equivalents of 3-mercaptopropionic acid. It is a colorless liquid at room temperature.[1]
Uses
Pentaerythritol tetrakis(3-mercaptopropionate) is a common thiol monomer reacted with alkenes in the thiol-ene reaction to form polymeric networks.[3] Being functionalized with four thiol groups, it can react with multifunctional alkenes to form thiol-ene networks.
References
- ↑ 1.0 1.1 "Pentaerythritol tetrakis(3-mercaptopropionate)". https://pubchem.ncbi.nlm.nih.gov/compound/82056.
- ↑ "MFCD00022104". http://www.chemspider.com/Chemical-Structure.74055.html.
- ↑ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition 49 (9): 1540–1573. doi:10.1002/anie.200903924.
 |

