Chemistry:Pentenoic acid
Pentenoic acid is any of five mono-carboxylic acids whose molecule has an unbranched chain of five carbons connected by three single bonds and one double bond. That is, any compound with one of the formulas HO(O=)C–CH=CH–CH
2–CH
3 (2-pentenoic), HO(O=)C–CH
2–CH=CH–CH
3 (3-pentenoic), or HO(O=)C–CH
2–CH
2–CH=CH
2 (4-pentenoic). In the IUPAC-recommended nomenclature, these acids are called pent-2-enoic, pent-3-enoic, and pent-4-enoic, respectively. All these compounds have the empirical formula C5H8O2.
Pentenoic acids are technically mono-unsaturated fatty acids, although they are rare or unknown in biological lipids (fats, waxes, phospholipids, etc.). A salt or ester of such an acid is called a pentenoate.
Geometric isomers
There are actually two 2-pentenoic acids, distinguished by the conformation of the two single C–C bonds adjacent to the double bond: either on the same side of the double bond's plane (cis or Z configuration) or on opposite sides of it (trans or E configuration).
Likewise, there are two 3-pentenoic acids. On the other hand, there is only one 4-pentenoic acid, since the two hydrogen atoms on the last carbon are symmetrically placed across the double bond's plane.
The full list of pentenoic acids is, therefore:
- cis-2-pentenoic or (2Z)-pent-2-enoic acid (CAS 16666-42-5, Nikkaji J97.998H, PUBchem 643793).[1][2]
- trans-2-pentenoic or (2E)-pent-2-enoic acid (CAS 13991-37-2, FDA 1RG66883CF, Nikkaji J97.997J, Beilstein 1720312, PUBchem 638122, JECFA 1804, FEMA 4193). MP ~10 °C; BP ~108 °C at 17 torr, ~198 °C; odor cheesy, sour. Occurs in banana, beer. Flavoring agent.[3]
- cis-3-pentenoic or (3Z)-pent-3-enoic acid (CAS 33698-87-2, Nikkaji J98.001C, PUBchem 5463134).[4]
- trans-3-pentenoic or (3E)-pent-3-enoic acid (BP ~52 °C at 4 torr) (CAS 1617-32-9, Nikkaji J98.000E, PUBchem 5282706). BP ~187 °C.[5][6]
- 4-pentenoic or pent-4-enoic acid, 3-vinylpropionic acid (CAS 591-80-0, FDA D4S77Y29FB, Nikkaji J53.731D, Beilstein 1633696, PUBchem 61138, JECFA 314, FEMA 2843). Dens ~0.975 at 25 °C; IoR ~1.428; MP ~ -23 °C; BP ~83 °C at 12 torr, ~188 °C; sol. water, slightly; odor cheese, mustard.[7] Toxic.[8][9][10]
Esters
Esters of pentenoic acids include:
- Ethyl cis-2-pentenoate (CAS 27805-84-1, Nikkaji J181.277G, PUBchem 11332473).[11]
- Ethyl trans-2-pentenoate (CAS 24410-84-2, Nikkaji J181.274B, PUBchem 5367761) BP ~150 °C.[12]
- Butyl 2-pentenoate (CAS 79947-84-5, Nikkaji J2.425.129B, PUBchem 5463534).[13]
- Ethyl trans-3-pentenoate (CAS 3724-66-1, Nikkaji J500.934K, PUBchem 5463078).[14]
- ethyl cis-3-pentenoate (CAS 27829-70-5, Nikkaji J746.757E, PUBchem 10997059).[15]
- isopropyl 3-pentenoate (CAS 62030-41-5, Nikkaji J746.761C, PUBchem 5463374).[16]
- Butyl 3-pentenoate (CAS 19825-93-5, PUBchem 5462980) Odor of chamomile. Flavoring agent.[17]
Derivatives
Some derivatives of pentenoic acid include:
- 2-Oxopent-4-enoic acid, transient species possibly produced by Azotobacter vinelandii
- 2-Amino-5-chloro-4-pentenoic acid, found in the mushroom Amanita cokeri
- 2-Methyl-3-pentenoic acid. Some esters are berry fruit flavors.[18]
- 2-Propyl-trans-2-pentenoic acid (2-en-valproic acid), major metabolite of anticonvulsant valproic acid.[19]
- cis-2-methyl-2-pentenoic acid 2-methyl-(2Z)-pent-2-enoic acid. (CAS 1617-37-4, FDA 07B8HQZ433, Nikkaji J421.583D, PUBchem 6436344) BP ~214 °C. Flavoring agent.[20]
- trans-2-Methyl-2-pentenoic acid 2-methyl-(2E)-pent-2-enoic acid. (CAS 16957-70-3, FDA 44I99E898B, Nikkaji J150.063E, PUBchem 5365909) dens ~0.987; IoR ~1.46; MP ~25 °C; BP ~124 °C at 30 torr, ~214 °C; odor fruity, strawberry. Flavoring agent.[21]
- 2-Methyl-2-pentenoic acid, cis/trans mix (CAS 3142-72-1, JECFA 1210, FEMA 3195, PUBchem 18458, US patent 3976801). Dens ~0.983 at 25 °C; IoR ~1.46; MP ~25 °C; BP ~124 °C at 30 torr, ~112 °C at 12 torr; sol. water, slightly; odor acidic, fruity, sweaty. Flavoring and perfuming agent.[22]
- 2-Methyl-4-pentenoic acid, 2-methyl-pent-4-enoic acid. The hexyl ester (CAS 58031-03-1, FDA MGQ3MUU64F, FEMA 3693, PUBchem 53426766, US patents 3966799,3976801) is a flavoring agent for chewing-gum, candy, beverages; .[23]
Gallery
| 4-Pentenoic acid | 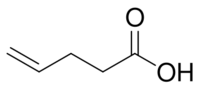
| |
| trans-2-pentenoic acid | 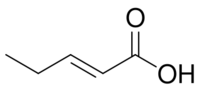
|
|
| 2-Oxo-4-pentenoic acid. | 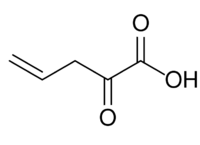
|
See also
- Valeric acid or pentanoic acid
- Pentynic acid
- Pentenedioic acid
- hexenoic acid
- Butenoic acid
References
- ↑ Regina Palkovits (2010): "Pentenoic acid pathways for cellulosic biofuels". Angewandte Chemie International Edition, volume 49, issue 26, pages 4336-4338. doi:10.1002/anie.201002061
- ↑ Perflavory (2020): "(Z)-2-pentenoic acid". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "(E)-2-pentenoic acid". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "(Z)-3-pentenoic acid". Accessed on 2020-08-27.
- ↑ Archibald M Hyson (1950): "Process for producing trans-3-pentenoic acid". US Patent 2586341. Filed on 1950-10-14, granted on 1952-02-19, assigned to EI Du Pont de Nemours; expired on 1969-02-19.
- ↑ Perflavory (2020): "(E)-3-pentenoic acid". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "4-pentenoic acid". Accessed on 2020-08-27.
- ↑ Allen M. Glasgow and H. Peter Chase (1975): "Production of the features of Reye's Syndrome in rats with 4-pentenoic acid". Pediatric Research, volume 9, pages 133-138. Quote: "4-Pentenoic acid, an analog of hypoglycin which is believed to cause Jamaican vomiting sickness, was administered intraperitoneally to rats in an attempt to produce the features of Reye's syndrome in rats." doi:10.1203/00006450-197503000-00005
- ↑ Allen M. Glasgow and H. Peter Chase (1975): "Inhibition of urea synthesis by 4-pentenoic acid". Biochemical and Biophysical Research Communications, volume 62, issue 2, pages 362-366. doi:10.1016/S0006-291X(75)80147-1
- ↑ Allen M. Glasgow and H. Peter Chase (1976): "Effect of pent-4-enoic acid, propionic acid and other short-chain fatty acids on citrulline synthesis in rat liver mitochondria.". Biochemical Journal, volume 156, issue 2, pages 301-307. Quote:"... pent-4-enoate inhibits citrulline synthesis by interfering with some aspect of mitochondrial energy metabolism. ... Propionate, butyrate and crotonate also inhibit mitochondrial citrulline synthesis, but much less than pent-4-enoate. ... Acetate, pentanoate, pent-2-enoate, hexanoate, octanoate, isovalerate, tiglylate and alpha-methylbutyrate have little or no effect on mitochondrial citrulline synthesis." doi:10.1042/bj1560301
- ↑ Perflavory (2020): "ethyl (Z)-2-pentenoate". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "ethyl (E)-2-pentenoate". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "butyl 2-pentenoate". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "ethyl (E)-3-pentenoate". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "ethyl (Z)-3-pentenoate". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "isopropyl 3-pentenoate". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "butyl 3-pentenoate". Accessed on 2020-08-27.
- ↑ Ching Y. Tseng, John B. Hall, Manfred Hugo Vock, Joaquin Vinals, and Edward J. Shuster (1975): "Flavoring with cis esters of 2-methyl-3-pentenoic acid". US Patent 2586341. Filed on 1975-05-09, granted on 1976-12-28, assigned to International Flavors and Fragrances Inc; expired on 1993-12-28.
- ↑ Wolfgang Löscher, Heinz Nau, Christian Marescaux, and Marguerite Vergnes (1984): "Comparative evaluation of anticonvulsant and toxic potencies of valproic acid and 2-en-valproic acid in different animal models of epilepsy". European Journal of Pharmacology, volume 99, issues 2–3, pages 211-218. doi:10.1016/0014-2999(84)90243-7
- ↑ Perflavory (2020): "(Z)-2-methyl-2-pentenoic acid". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "(E)-2-methyl-2-pentenoic acid". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "2-methyl-2-pentenoic acid (E/Z mix)". Accessed on 2020-08-27.
- ↑ Perflavory (2020): "hexyl 2-methyl-4-pentenoate". Accessed on 2020-08-27.
 |

