Chemistry:Perifosine
| |
| |
| Names | |
|---|---|
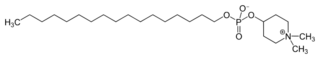
| IUPAC name
1,1-Dimethylpiperidinium-4-yl octadecyl phosphate
| |
| Other names
D 21266; KRX 0401
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C25H52NO4P | |
| Molar mass | 461.668 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perifosine (also KRX-0401) is a former drug candidate that was under development for a variety of cancer indications. It is an alkyl-phospholipid[1] structurally related to miltefosine. Perifosine interrupts the PI3K/AKT/mTOR pathway by acting as an allosteric AKT inhibitor targeting the pleckstrin homology domain of AKT.[2] It was being developed by Keryx Biopharmaceuticals who had licensed it from Æterna Zentaris Inc.[3]
In 2010, perifosine received orphan drug status in the U.S. for the treatment of multiple myeloma and neuroblastoma, and for multiple myeloma in the EU.[4] However, both were later withdrawn.[5][6]
In 2011 it was in a phase III trial for colorectal cancer,[7] and another for multiple myeloma.[4][8] On April 2, 2012, it was announced that perifosine failed its phase III clinical trial for treatment of colon cancer.[9] Detailed results were released in June 2012.[10] On March 11, 2013 Aeterna Zentaris announced the discontinuing of Phase 3 clinical trial of perifosine for the treatment of relapsed and refractory multiple myeloma.[11]
References
- ↑ Kondapaka (Nov 2003). "Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation". Molecular Cancer Therapeutics (Mol Cancer Ther) 2 (11): 1093–103. PMID 14617782. http://mct.aacrjournals.org/content/2/11/1093.full.
- ↑ Keane, N. A.; Glavey, S. V.; Krawczyk, J.; O'Dwyer, M. (2014). "AKT as a therapeutic target in multiple myeloma". Expert Opinion on Therapeutic Targets 18 (8): 897–915. doi:10.1517/14728222.2014.924507. PMID 24905897.
- ↑ Smartoncology newsletter , Feb 2010
- ↑ 4.0 4.1 "Yakult Pays Aeterna Zentaris $8.3M for Japanese Rights to Pivotal-Stage Cancer Drug". 9 March 2011. http://www.genengnews.com/gen-news-highlights/yakult-pays-aeterna-zentaris-8-3m-for-japanese-rights-to-pivotal-stage-cancer-drug/81244795/.
- ↑ "FDA Orphan Drug Designations and Approvals: Perifosine". https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=310010.
- ↑ "EU/3/10/740: Orphan designation for the treatment of multiple myeloma: Perifosine". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu310740.
- ↑ "Perifosine Plus Capecitabine Versus Placebo Plus Capecitabine in Patients with Refractory Advanced Colorectal Cancer - Full Text View - ClinicalTrials.gov". http://www.clinicaltrial.gov/ct2/show/NCT01097018.
- ↑ Clinical trial number NCT01002248 for "Assessment of Efficacy and Safety of Perifosine, Bortezomib and Dexamethasone in Multiple Myeloma Patients" at ClinicalTrials.gov
- ↑ "Aeterna Zentaris Regains North American Rights to Akt Inhibitor from Keryx". May 2012. http://www.genengnews.com/gen-news-highlights/aeterna-zentaris-regains-north-american-rights-to-akt-inhibitor-from-keryx/81246731/.
- ↑ "Aeterna Zentaris: Phase 3 Data for Perifosine in Colorectal Cancer Presented at ASCO Meeting". 4 June 2012. http://www.aezsinc.com/en/page.php?p=60&q=508.
- ↑ "Æterna Zentaris Inc". http://www.aezsinc.com/en/page.php?p=60&q=550.
 |
