Chemistry:Phosphorolysis
Phosphorolysis is the cleavage of a compound in which inorganic phosphate is the attacking group. It is analogous to hydrolysis.[1] An example of this is glycogen breakdown by glycogen phosphorylase, which catalyzes attack by inorganic phosphate on the terminal glycosyl residue at the nonreducing end of a glycogen molecule. If the glycogen chain has n glucose units, the products of a single phosphorolytic event are one molecule of glucose 1-phosphate and a glycogen chain of n-1 remaining glucose units.
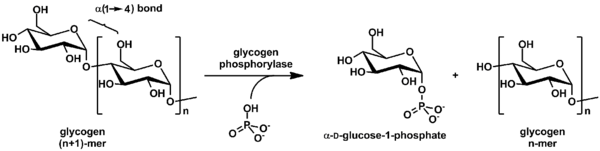
In addition, sometimes phosphorolysis is preferable to hydrolysis (like in the breakdown of glycogen or starch, as in the example above) because glucose 1-phosphate yields more ATP than does free glucose when subsequently catabolized to pyruvate.
Another example of phosphorolysis is seen in the conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate in glycolysis. The mechanism involves phosphorolysis.
See also
References
- ↑ Stryer, L. (1988) Biochemistry, 3rd ed., Freeman (p. 451)
External links
 |

