Chemistry:Phytosphingosine
From HandWiki
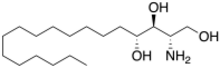
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2S,3S,4R)-2-Aminooctadecane-1,3,4-triol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1725301 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H39NO3 | |
| Molar mass | 317.514 g·mol−1 |
| Melting point | 102–103 °C (216–217 °F; 375–376 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H318, H410 | |
| P273, P280, P305+351+338, P310, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phytosphingosine is a sphingoid base, a fundamental building block of more complex sphingolipids. It is abundant in plants and fungi and present in animals.[1] Phytosphingosine has also been found to have interesting T-cell related anti-inflammatory properties in models of inflammatory bowel disease.[2]
References
- ↑ Park, Moon-Taek; Kang, Jung A.; Choi, Jung-A.; Kang, Chang-Mo; Kim, Tae-Hwan; Bae, Sangwoo; Kang, Seongman; Kim, Sujong et al. (February 2003). "Phytosphingosine induces apoptotic cell death via caspase 8 activation and Bax translocation in human cancer cells". Clinical Cancer Research 9 (2): 878–885. ISSN 1078-0432. PMID 12576463.
- ↑ Montenegro-Burke, J. Rafael; Kok, Bernard P.; Guijas, Carlos; Domingo-Almenara, Xavier; Moon, Clara; Galmozzi, Andrea; Kitamura, Seiya; Eckmann, Lars et al. (2021-09-28). "Metabolomics activity screening of T cell–induced colitis reveals anti-inflammatory metabolites" (in en). Science Signaling 14 (702): eabf6584. doi:10.1126/scisignal.abf6584. ISSN 1945-0877. PMID 34582249. PMC 8757460. https://www.science.org/doi/10.1126/scisignal.abf6584.
 |

