Chemistry:Poly(ethylene succinate)
| |
| Identifiers | |
|---|---|
| ChemSpider |
|
| Properties | |
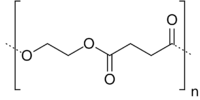
| (C6H8O4)n | |
| Melting point | 103–106 °C (217–223 °F; 376–379 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Poly(ethylene succinate) (PES) is an aliphatic synthetic polyester with a melting point from 103–106 °C. It is synthesized from dicarboxylic acids; either by ring-opening polymerization of succinic anhydride with ethylene oxide or by polycondensation of succinic acid and ethylene glycol.[1] Thermophilic Bacillus sp. TT96 is found in soil and can degrade PES. Mesophilic PES degrading microorganisms were found in the Bacillus and Paenibacillus species; strain KT102; a relative of Bacillus pumilus was the most capable of degrading PES film. The fungal species NKCM1003 a type of Aspergillus clavatus also degrades PES film. The solubility of lithium salts (e.g. lithium perchlorate, LiClO4) in PES made it a good alternative to poly(ethylene oxide) (PEO) during early development of solid polymer electrolytes for lithium ion batteries.[2]
References
- ↑ Yutaka Tokiwa; Buenaventurada P. Calabia; Charles U. Ugwu; Seiichi Aiba (September 2009). "Biodegradability of Plastics". International Journal of Molecular Sciences 10 (9): 3722–3742. doi:10.3390/ijms10093722. PMID 19865515.
- ↑ Watanabe, Masayoshi; Rikukawa, Masahiro; Sanui, Kohei; Ogata, Naoya; Kato, Hisaaki; Kobayashi, Tadahiko; Ohtaki, Zentaro (1984). "Ionic conductivity of polymer complexes formed by poly(ethylene succinate) and lithium perchlorate" (in EN). Macromolecules 17 (12): 2902–2908. doi:10.1021/ma00142a078. ISSN 0024-9297. Bibcode: 1984MaMol..17.2902W.
 |

