Chemistry:Polyol
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups (–OH). The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols,[1] triols,[2] and tetrols,[3][4] respectively.
Classification
Polyols may be classified according to their chemistry.[5] Some of these chemistries are polyether, polyester,[6] polycarbonate[7][8] and also acrylic polyols.[9][10] Polyether polyols may be further subdivided and classified as polyethylene oxide or polyethylene glycol (PEG), polypropylene glycol (PPG) and Polytetrahydrofuran or PTMEG. These have 2, 3 and 4 carbons respectively per oxygen atom in the repeat unit. Polycaprolactone polyols are also commercially available.[11] There is also an increasing trend to use biobased (and hence renewable) polyols.[12][13][14][15]
Uses
Polyether polyols have numerous uses.[16][17] As an example, polyurethane foam is a big user of polyether polyols.[18]
Polyester polyols can be used to produce rigid foam.[19][20] They are available in both aromatic and aliphatic versions.[21][22] They are also available in mixed aliphatic-aromatic versions often made from recycled raw materials, typically polyethylene terephthalate (PET).[23]
Acrylic polyols are generally used in higher performance applications where stability to ultraviolet light is required[24] and also lower VOC coatings.[25][26] Other uses include direct to metal coatings.[27] As they are used where good UV resistance is required, such as automotive coatings, the isocyanate component also tends to be UV resistant and hence isocyanate oligomers or prepolymers based on Isophorone diisocyanate are generally used.[28]
Caprolactone-based polyols produce polyurethanes with enhanced hydrolysis resistance.[29][30]
Polycarbonate polyols are more expensive than other polyols and are thus used in more demanding applications.[31][32] They have been used to make an isophorone diisocyanate based prepolymer which is then used in glass coatings.[33] They may be used in reactive hotmelt adhesives.[34]
All polyols may be used to produce polyurethane prepolymers.[35][36][37] These then find use in coatings,[38] adhesives, sealants and elastomers.[39]
Low molecular weight polyols
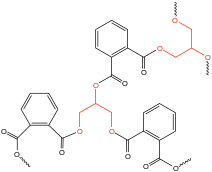
Low molecular weight polyols are widely used in polymer chemistry where they function as crosslinking agents and chain extenders. Alkyd resins for example, use polyols in their synthesis and are used in paints and in molds for casting. They are the dominant resin or "binder" in most commercial "oil-based" coatings. Approximately 200,000 tons of alkyd resins are produced each year. They are based on linking reactive monomers through ester formation. Polyols used in the production of commercial alkyd resins are glycerol, trimethylolpropane, and pentaerythritol.[40] In polyurethane prepolymer production, a low molecular weight polyol-diol such as 1,4-butanediol may be used as a chain extender to further increase molecular weight though it does increase viscosity because more hydrogen bonding is introduced.[38]
| Low molecular weight polyols |
Pentaerythritol |
 |
Sugar alcohols
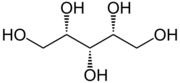
Sugar alcohols, a class of low molecular weight polyols, are commonly obtained by hydrogenation of sugars.[41]: 363 They have the formula (CHOH)nH2, where n = 4–6.[42]
Sugar alcohols are added to foods because of their lower caloric content than sugars; however, they are also, in general, less sweet, and are often combined with high-intensity sweeteners. They are also added to chewing gum because they are not broken down by bacteria in the mouth or metabolized to acids, and thus do not contribute to tooth decay. Maltitol, sorbitol, xylitol, erythritol, and isomalt are common sugar alcohols.
Polymeric polyols
| Polymeric polyols |
 Polyether polyol (The oxygen atoms of the ether linkages are shown in blue.) |
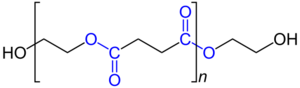 Polyester polyol (The oxygen and carbon atoms of the ester groups are shown in blue.) |
The term polyol is used for various chemistries of the molecular backbone. Polyols may be reacted with diisocyanates or polyisocyanates to produce polyurethanes. MDI finds considerable use in PU foam production.[43] Polyurethanes are used to make flexible foam for mattresses and seating, rigid foam insulation for refrigerators and freezers, elastomeric shoe soles, fibers (e.g. Spandex), coatings, sealants and adhesives.[44]
The term polyol is also attributed to other molecules containing hydroxyl groups. For instance, polyvinyl alcohol is (CH2CHOH)n with n hydroxyl groups where n can be in the thousands. Cellulose is a polymer with many hydroxyl groups, but it is not referred to as a polyol.
Polyols from recycled or renewable sources
There are polyols based on renewable sources such as plant-based materials including castor oil and cottonseed oil.[45][46][47] Vegetable oils and biomass are also potential renewable polyol raw materials.[48] Seed oil can even be used to produce polyester polyols.[49]
Properties
Since the generic term polyol is only derived from chemical nomenclature and just indicates the presence of several hydroxyl groups, no common properties can be assigned to all polyols. However, polyols are usually viscous at room temperature due to hydrogen bonding.
See also
- Cyclitol
- Oligomer
- Polyurethane
References
- ↑ "Basic IUPAC Organic Nomenclature - Diols (or polyols)". 2022. https://www.chem.ucalgary.ca/courses/351/WebContent/orgnom/alcohols/alcohols-02.html.
- ↑ "Definition of TRIOL" (in en). https://www.merriam-webster.com/dictionary/triol.
- ↑ "Tetrol Meaning". https://www.yourdictionary.com/tetrol.
- ↑ PubChem. "Butane-1,2,3,4-tetrol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8998.
- ↑ Howarth, GA (2003). "Polyurethanes, polyurethane dispersions and polyureas: Past, present and future". Surface Coatings International Part B: Coatings Transactions 86 (2): 111–118. doi:10.1007/BF02699621.
- ↑ "Polyester Polyols - an overview". https://www.sciencedirect.com/topics/engineering/polyester-polyols.
- ↑ Scharfenberg, Markus; Hofmann, Silja; Preis, Jasmin; Hilf, Jeannette; Frey, Holger (2017-08-22). "Rigid Hyperbranched Polycarbonate Polyols from CO2 and Cyclohexene-Based Epoxides". Macromolecules 50 (16): 6088–6097. doi:10.1021/acs.macromol.7b01276. ISSN 0024-9297. https://doi.org/10.1021/acs.macromol.7b01276.
- ↑ , Steven; Shawn Brown & Mark Sonnenschein et al."Polycarbonate polyols and polyurethanes made therefrom" patent WO2011129940A1, issued 2011-10-20
- ↑ Roesler, Richard (26 March 1986). "Acrylic polyols having low residual monomer content European Patent". https://patentimages.storage.googleapis.com/78/38/fb/cd9c639d25a624/EP0197460A2.pdf.
- ↑ "Polyacrylate Polyols". https://ebrary.net/14329/environment/polyacrylate_polyols.
- ↑ "Polycaprolactone Polyols Market Report - Size and Share by 2026 | AMR" (in en). https://www.alliedmarketresearch.com/polycaprolactone-polyols-market.
- ↑ Li, Yonghui; Sun, Xiuzhi Susan (2015-05-15). "Synthesis and characterization of acrylic polyols and polymers from soybean oils for pressure-sensitive adhesives" (in en). RSC Advances 5 (55): 44009–44017. doi:10.1039/C5RA04399A. ISSN 2046-2069. https://pubs.rsc.org/en/content/articlelanding/2015/ra/c5ra04399a.
- ↑ "Bio-acrylic polyols for two pack polyurethane coating" (in en). Journal of Scientific and Industrial Research 63 (3): 259–264. March 2004. https://www.researchgate.net/publication/268398112. Retrieved 2022-02-13.
- ↑ Kasprzyk, Paulina; Sadowska, Ewelina; Datta, Janusz (2019-11-01). "Investigation of Thermoplastic Polyurethanes Synthesized via Two Different Prepolymers" (in en). Journal of Polymers and the Environment 27 (11): 2588–2599. doi:10.1007/s10924-019-01543-7. ISSN 1572-8919.
- ↑ Gurunathan, T.; Mohanty, Smita; Nayak, Sanjay K. (2015-03-01). "Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization" (in en). Progress in Organic Coatings 80: 39–48. doi:10.1016/j.porgcoat.2014.11.017. ISSN 0300-9440. https://www.sciencedirect.com/science/article/pii/S0300944014003725.
- ↑ Datta, Janusz; Kosiorek, Paulina; Włoch, Marcin (2017-04-01). "Synthesis, structure and properties of poly(ether-urethane)s synthesized using a tri-functional oxypropylated glycerol as a polyol" (in en). Journal of Thermal Analysis and Calorimetry 128 (1): 155–167. doi:10.1007/s10973-016-5928-2. ISSN 1588-2926.
- ↑ Kantheti, Sasidhar; Sarath, P. S.; Narayan, Ramanuj; Raju, K. V. S. N. (2013-12-01). "Synthesis and characterization of triazole rich polyether polyols using click chemistry for highly branched polyurethanes" (in en). Reactive and Functional Polymers 73 (12): 1597–1605. doi:10.1016/j.reactfunctpolym.2013.09.002. ISSN 1381-5148. https://www.sciencedirect.com/science/article/pii/S138151481300206X.
- ↑ Abraham, T.W.; Höfer, R. (2012). "10.03 - Lipid-Based Polymer Building Blocks and Polymers". Polymer Science: A Comprehensive Reference. Elsevier. pp. 15–58. doi:10.1016/B978-0-444-53349-4.00253-3. ISBN 9780080878621.
- ↑ McAdams, Carina; Farmer, Steven (September 2003). "Stabilization of Rigid Systems Containing Aromatic Polyester Polyol and Water". Journal of Cellular Plastics 39 (September 2003): 369–386. doi:10.1177/0021955X03035067.
- ↑ "Polyester polyols for rigid foam". February 2022. https://www.stepan.com/content/dam/stepan-dot-com/webdam/website-product-documents/literature/polyester-polyols/StepanRigidFoamBrochure.pdf.
- ↑ "Aromatic Polyester Polyols" (in en). https://purinova.com/en/products/polyester-polyols/aromatic-polyester-polyols.
- ↑ "Polyester Polyols". May 2018. https://www.nord-composites.com/fichiersusers/files/NORD-COMPOSITES_BrochuresPolyesterPolyols_May_2018.pdf.
- ↑ Makuska, Ricardas (2008). "Glycolysis of industrial poly(ethylene terephthalate) waste directed to bis(hydroxyethylene) terephthalate and aromatic polyester polyols". Chemija 19 (2): 29–34. https://mokslozurnalai.lmaleidykla.lt/publ/0235-7216/2008/2/29-34.pdf.
- ↑ , Wei & Stephen H. Harris"Preparation of acrylic polyols" patent US6762262B1, issued 2004-07-13
- ↑ Ionescu, Mihail (2019). "10. Acrylic polyols" (in en). Aromatic Polyester Polyols: Chemistry and Technology. 1. De Gruyter. pp. 267–272. doi:10.1515/9783110644104-010. ISBN 978-3-11-064410-4.
- ↑ "New Acrylic Polyols for Low-VOC Coatings" (in en). 2002-05-31. https://www.pcimag.com/articles/84259-new-acrylic-polyols-for-low-voc-coatings.
- ↑ "Acrylic polyol with enhanced performance for 2K PUR direct-to-metal coatings". BASF. https://insights.basf.com/home/article/read/acrylic-polyol-with-enhanced-performance-for-2k-pur-direct-to-metal-coatings.
- ↑ Gite, V. V.; Mahulikar, P. P.; Hundiwale, D. G. (2010-08-01). "Preparation and properties of polyurethane coatings based on acrylic polyols and trimer of isophorone diisocyanate" (in en). Progress in Organic Coatings 68 (4): 307–312. doi:10.1016/j.porgcoat.2010.03.008. ISSN 0300-9440. https://www.sciencedirect.com/science/article/pii/S030094401000086X.
- ↑ Takaaki, Fujiwa (19 July 1990). "A polycaprolactone polyol and hydrolysis resistant polyurethane resins prepared therefrom patent 0 409 735 A1". https://patentimages.storage.googleapis.com/8b/3e/79/e4bdbb854918b7/EP0409735A1.pdf.
- ↑ Huang, Shan; Xiao, Juan; Zhu, Yan’an; Qu, Jinqing (2017-05-01). "Synthesis and properties of spray-applied high solid content two component polyurethane coatings based on polycaprolactone polyols" (in en). Progress in Organic Coatings 106: 60–68. doi:10.1016/j.porgcoat.2017.02.011. ISSN 0300-9440. https://www.sciencedirect.com/science/article/pii/S030094401630786X.
- ↑ Pohl, M.; Danieli, E.; Leven, M. et al. (2016-12-13). "Dynamics of Polyether Polyols and Polyether Carbonate Polyols" (in en). Macromolecules 49 (23): 8995–9003. doi:10.1021/acs.macromol.6b01601. ISSN 0024-9297. https://pubs.acs.org/doi/10.1021/acs.macromol.6b01601.
- ↑ "Polycarbonate Diols for Ultimate Performance Polyurethanes" (in en-us). https://www.gantrade.com/blog/ultimate-performance-polyurethanes-based-on-polycarbonate-diols.
- ↑ Wilson, Michael G. (November 1991). "New coatings for glass". Journal of the Oil and Colour Chemists Association 11: 412–415.
- ↑ Cherian, Anna (2014-11-01). "Carbon Dioxide-Based Polycarbonate Polyols for Polyurethane Systems" (in en). https://www.adhesivesmag.com/articles/93368-carbon-dioxide-based-polycarbonate-polyols-for-polyurethane-systems.
- ↑ Harani, H.; Fellahi, S.; Bakar, M. (1998). "Toughening of epoxy resin using synthesized polyurethane prepolymer based on hydroxyl-terminated polyesters" (in en). Journal of Applied Polymer Science 70 (13): 2603–2618. doi:10.1002/(SICI)1097-4628(19981226)70:13<2603::AID-APP6>3.0.CO;2-4. ISSN 1097-4628.
- ↑ Shi, Minxian; Zheng, Juanli; Huang, Zhixiong; Qin, Yan (2011-03-01). "Synthesis of Polyurethane Prepolymers and Damping Property of Polyurethane/Epoxy Composites". Advanced Science Letters 4 (3): 740–744. doi:10.1166/asl.2011.1597. https://www.ingentaconnect.com/contentone/asp/asl/2011/00000004/00000003/art00020.
- ↑ Pokharel, Pashupati; Lee, Dai Soo (2014-10-01). "High performance polyurethane nanocomposite films prepared from a masterbatch of graphene oxide in polyether polyol" (in en). Chemical Engineering Journal 253: 356–365. doi:10.1016/j.cej.2014.05.046. ISSN 1385-8947. https://www.sciencedirect.com/science/article/pii/S1385894714006214.
- ↑ 38.0 38.1 Howarth, G.A. (2000). "Legislation‐compliant polyurethane and epoxy coatings". Pigment & Resin Technology 29 (6): 325–336. doi:10.1108/03699420010355120.
- ↑ Wang, Lei; Shen, Yiding; Lai, Xiaojuan et al. (2011-05-01). "Synthesis and properties of crosslinked waterborne polyurethane" (in en). Journal of Polymer Research 18 (3): 469–476. doi:10.1007/s10965-010-9438-9. ISSN 1572-8935. https://doi.org/10.1007/s10965-010-9438-9.
- ↑ Frank N. Jones. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_409.
- ↑ Malani, Ritesh S.; Malshe, Vinod C.; Thorat, Bhaskar Narayan (2022). "Polyols and polyurethanes from renewable sources: past, present, and future—part 2: plant-derived materials" (in en). Journal of Coatings Technology and Research 19 (2): 361–375. doi:10.1007/s11998-021-00534-5. ISSN 1935-3804. https://doi.org/10.1007/s11998-021-00534-5.
- ↑ Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2012. doi:10.1002/14356007.a25_413.pub3. ISBN 978-3527306732.
- ↑ "4,4′-Methylene diphenyl isocyanate (MDI) and polymeric MDI″ (PMDI) [MAK Value Documentation, 1997"] (in en), The MAK-Collection for Occupational Health and Safety (John Wiley & Sons, Ltd): pp. 66–96, 2012, doi:10.1002/3527600418.mb10168stae0008, ISBN 978-3-527-60041-0, https://onlinelibrary.wiley.com/doi/abs/10.1002/3527600418.mb10168stae0008, retrieved 2022-02-12
- ↑ Boustead, I. (2005). "Polyurethane rigid foam". Eco-Profiles of the European Plastics Industry. Brussels: PlasticsEurope. Archived from the original on 2013-09-25. https://web.archive.org/web/20130925082609/http://www.isopa.org/isopa/uploads/Documents/documents/rigid%20foam%20LCI.pdf.
- ↑ Nelson, Thomas J.; Masaki, Bryan; Morseth, Zachary; Webster, Dean C. (2013-11-01). "Highly functional biobased polyols and their use in melamine–formaldehyde coatings" (in en). Journal of Coatings Technology and Research 10 (6): 757–767. doi:10.1007/s11998-013-9524-0. ISSN 1935-3804. https://doi.org/10.1007/s11998-013-9524-0. Retrieved 2023-03-09.
- ↑ Jia, Lian Kun; Gong, Li Xiang; Ji, Wen Jiao; Kan, Cheng You (2011-11-01). "Synthesis of vegetable oil based polyol with cottonseed oil and sorbitol derived from natural source" (in en). Chinese Chemical Letters 22 (11): 1289–1292. doi:10.1016/j.cclet.2011.05.043. ISSN 1001-8417. https://www.sciencedirect.com/science/article/pii/S1001841711001860.
- ↑ Narute, Prashant; Palanisamy, Aruna (2016-01-01). "Study of the performance of polyurethane coatings derived from cottonseed oil polyol" (in en). Journal of Coatings Technology and Research 13 (1): 171–179. doi:10.1007/s11998-015-9741-9. ISSN 1935-3804. https://doi.org/10.1007/s11998-015-9741-9.
- ↑ Malani, Ritesh S.; Malshe, Vinod C.; Thorat, Bhaskar Narayan (2022). "Polyols and polyurethanes from renewable sources: past, present and future—part 1: vegetable oils and lignocellulosic biomass" (in en). Journal of Coatings Technology and Research 19 (1): 201–222. doi:10.1007/s11998-021-00490-0. ISSN 1935-3804. https://doi.org/10.1007/s11998-021-00490-0.
- ↑ Argyropoulos, John; Popa, Paul; Spilman, Gary; Bhattacharjee, Debkumar; Koonce, William (2009-12-01). "Seed oil based polyester polyols for coatings" (in en). Journal of Coatings Technology and Research 6 (4): 501–508. doi:10.1007/s11998-008-9154-0. ISSN 1935-3804. https://doi.org/10.1007/s11998-008-9154-0. Retrieved 2023-03-14.
External links
 |
