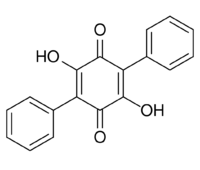
Chemistry:Polyporic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
23,26-Dihydroxy[11,21:24,31-terphenyl]-22,25-dione | |
| Other names
Polyporin; Orygameic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | C118527 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H12O4 | |
| Molar mass | 292.290 g·mol−1 |
| Hazards | |
| Main hazards | Toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polyporic acid is a para-terphenyl benzoquinone compound first identified by German chemist Stahlschmidt from a mycelial culture of the fungus species Hapalopilus nidulans in 1877.[1][2] This chemical, present at 20–40% of the fresh weight of the fruit bodies,[3] inhibits the enzyme dihydroorotate dehydrogenase.[4] It is found in other mushrooms, but in much lower amounts.[4]
In animal studies, consumption of polyporic acid caused reduced locomotor activity, depressed visual placing response, hepatorenal failure, metabolic acidosis, hypokalaemia, and hypocalcaemia.[4] Because these effects are similar to those observed in individuals poisoned by H. nidulans, polyporic acid is thought to be the primary toxin in H. nidulans.[4]
Polyporic acid has some antifungal[5] and antibacterial activity.[6] It has been shown to be an intermediate in the biosynthesis of allantofuranone, a gamma-lactone antibiotic from the fungus Allantophomopsis lycopodina.[7]
References
- ↑ Stahlschmidt C. (1877). "Ueber eine neue in der Natur vorkommende organische Säure". Justus Liebigs Annalen der Chemie 187 (2–3): 177–197. doi:10.1002/jlac.18771870204. https://zenodo.org/record/1447337.
- ↑ Polyhydroxy-p-terphenyls and related p-terphenylquinones from fungi: overview and biological properties. 29. 2003. 263–307. doi:10.1016/S1572-5995(03)80009-1. ISBN 9780444515100.
- ↑ Räisänen R. (2009). "Dyes from lichens and mushrooms". Handbook of Natural Colorants. Chichester, UK: John Wiley & Sons. p. 192. ISBN 978-0-470-74496-3. https://books.google.com/books?id=hBFxuH5uXyIC&pg=PA192.
- ↑ 4.0 4.1 4.2 4.3 "Biological effects of the dihydroorotate dehydrogenase inhibitor polyporic acid, a toxic constituent of the mushroom Hapalopilus rutilans, in rats and humans". Archives of Toxicology 72 (11): 711–721. 1998. doi:10.1007/s002040050565. PMID 9879809.
- ↑ "The effect on fungal growth of some 2,5-dihydroxy-1,4-benzoquinones". Canadian Journal of Microbiology 23 (7): 845–51. 1977. doi:10.1139/m77-126. PMID 884625.
- ↑ "The antibacterial activity of some naturally occurring 2,5-dihydroxy-1,4-benzoquinones". Canadian Journal of Microbiology 30 (8): 1068–1092. 1984. doi:10.1139/m84-166. PMID 6541963.
- ↑ "Elucidation of the biosynthesis and degradation of allantofuranone by isotopic labelling and fermentation of modified precursors". ChemBioChem 12 (1): 148–154. 2011. doi:10.1002/cbic.201000448. PMID 21181846.
 |

