Chemistry:Terphenyl

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
11,21:24,31-Terphenyl[1] | |
| Other names
1,1':4',1''-Terphenyl[1]
p-Terphenyl 1,4-Diphenylbenzene para-Diphenylbenzene p-Diphenylbenzene para-Triphenyl p-Triphenyl | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 1908447 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| |
| |
| Properties | |
| C18H14 | |
| Molar mass | 230.310 g·mol−1 |
| Appearance | White powder[2] |
| Density | 1.24 g/cm3 |
| Melting point | 212 to 214 °C (414 to 417 °F; 485 to 487 K)[2] 212-213 °C[4] |
| Boiling point | 389 °C (732 °F; 662 K)[4] |
| Insoluble[2] | |
Refractive index (nD)
|
1.65[3] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H315, H319, H335, H400 | |
| P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 207 °C (405 °F; 480 K)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
C 9 mg/m3 (1 ppm)[5][6][7] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
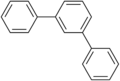
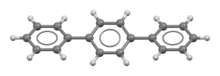
Terphenyls are a group of closely related aromatic hydrocarbons. Also known as diphenylbenzenes or triphenyls, they consist of a central benzene ring substituted with two phenyl groups. There are three substitution patterns: ortho-terphenyl, meta-terphenyl, and para-terphenyl. Commercial grade terphenyl is generally a mixture of the three isomers. This mixture is used in the production of polychlorinated terphenyls, which were formerly used as heat storage and transfer agents.[2]
p-Terphenyl derivatives are found in various fungi and bacteria. One example is atromentin, a pigment found in some mushrooms. These natural p-terphenyls are better described as diphenylquinones or diphenylhydroquinones. Some m-terphenyl compounds occur in plants.[8]
-
ortho-Terphenyl
-
meta-Terphenyl
-
para-Terphenyl
See also
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 345. doi:10.1039/9781849733069-00130. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 p-Terphenyl at chemicalland21.com
- ↑ "Organic molecular single crystals". cryos-beta.kharkov.ua. http://www.cryos-beta.kharkov.ua/organic.php.
- ↑ 4.0 4.1 4.2 p-Terphenyl at Sigma-Aldrich
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0591". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0591.html.
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0592". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0592.html.
- ↑ NIOSH Pocket Guide to Chemical Hazards. "#0593". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0593.html.
- ↑ Liu, Ji-Kai (2006). "Natural Terphenyls: Developments since 1877". Chemical Reviews 106 (6): 2209–2223. doi:10.1021/cr050248c. PMID 16771447.
External links
- p-Terphenyl at the Oregon Laser Medical Center
- o-Terphenyl, m-Terphenyl, p-Terphenyl at Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health
 |