Chemistry:Polytelluride
In chemistry, a polytelluride usually refers to anions of the formula (Ten)2-. Many main group and transition metals form complexes with polytelluride anions.[1]
Preparation
Conceptually, polytellurides are derived from polytelluranes H2Ten, but such neutral species are not known (even H2Te is labile). Instead, analogous to the preparation of many Zintl ions, polytellurides are produced by reduction of elemental Te with alkali metals. Such reactions can be conducted by heating a mixture of the solids or by dissolving Te metal in amine solvents of alkali metals. Once generated, these alkali metal polytellurides can be converted to lipophilic salts by treatment cryptand ligands or by ion exchange with quat salts.
Structures
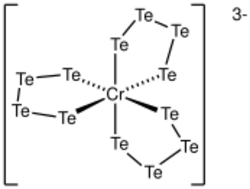
Salts of polytellurides have often been characterized by X-ray crystallography. Polytelluride salts generally feature open chains, which adopt a zig-zag conformation. In some cases, cyclic structures are observed as in Li2Te7, which features a square-planar Te center bound to two Te-Te-Te chains.
As ligands in coordination complexs, polytellurides are generally bidentate. Complexes of penta-, tetra-, and tritelluride ligands are known. One example is the spirocyclic [Zn(Te4)2]2-.[3]
See also
Further reading
- Graf, Christian; Assoud, Abdeljalil; Mayasree, Oottil; Kleinke, Holger (2009). "Solid State Polyselenides and Polytellurides: A Large Variety of Se–Se and Te–Te Interactions". Molecules 14 (9): 3115–3131. doi:10.3390/molecules14093115. PMID 19783911.
- Sheldrick, William S. (2012). "Polychalcogenide Anions: Structural Diversity and Ligand Versatility". Zeitschrift für Anorganische und Allgemeine Chemie 638 (15): 2401–2424. doi:10.1002/zaac.201200241.
- Smith, Donna M.; Ibers, James A. (2000). "Syntheses and Solid-State Structural Chemistry of Polytelluride Anions". Coordination Chemistry Reviews 200-202: 187–205. doi:10.1016/S0010-8545(00)00256-3.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 763-765. ISBN 978-0-08-037941-8.
- ↑ Sekar, Perumal; Ibers, James A. (2002). "Synthesis and Structure of [PPh4]3[Cr(Te4)3]·DMF: The First Tris(tetratelluride) Complex". Inorganic Chemistry 41 (3): 450–451. doi:10.1021/ic015585s.
- ↑ Kanatzidis, Mercouri G. (1990). "Soluble Polychalcogenides of the Late Transition and Main Group Elements". Comments on Inorganic Chemistry 10 (4–5): 161–195. doi:10.1080/02603599008048650.
 |


