Chemistry:Ponceau S
| |
| Names | |
|---|---|
| IUPAC name
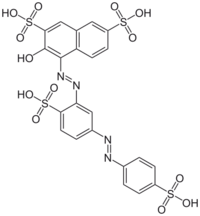
tetrasodium;3-hydroxy-4-[[2-sulfonato-4-[(4-sulfonatophenyl)diazenyl]phenyl]diazenyl]naphthalene-2,7-disulfonate
| |
| Other names
Acid red 112
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C22H16N4O13S4 | |
| Molar mass | 672.63 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
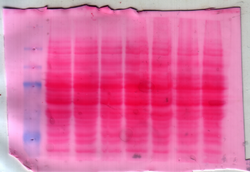
Ponceau S, Acid Red 112, or C.I. 27195 (systematic name: 3-hydroxy-4-(2-sulfo-4-[4-sulfophenylazo]phenylazo)-2,7-naphthalenedisulfonic acid sodium salt[1]) is a sodium salt of a diazo dye of a light red color, that may be used to prepare a stain for rapid reversible detection of protein bands on nitrocellulose or polyvinylidene fluoride (PVDF) membranes (western blotting), as well as on cellulose acetate membranes.[2] A Ponceau S stain is useful because it does not appear to have a deleterious effect on the sequencing of blotted polypeptides and is therefore one method of choice for locating polypeptides on western blots for blot-sequencing.[2] It is also easily reversed with water washes, facilitating subsequent immunological detection.[2] The stain can be completely removed from the protein bands by continued washing.[2] Common stain formulations include 0.1% (w/v) Ponceau S in 5% acetic acid or 2% (w/v) Ponceau S in 30% trichloroacetic acid and 30% sulfosalicylic acid.[3]
See also
References
- ↑ "Ponceau S solution". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/sial/p3504?lang=en. Retrieved 2016-11-24.
- ↑ 2.0 2.1 2.2 2.3 Al-Amoudi, M. S.; Salman, Mahmoud; Al-Majthoub, M. M.; Adam, Abdel Majid A.; Alshanbari, Naif A.; Refat, Moamen S. (9 October 2013). "Spectral studies to increase the efficiency and stability of laser dyes by charge-transfer reactions for using in solar cells: charge-transfer complexes of Ponceau S with p-chloranil, chloranilic and picric acids". Research on Chemical Intermediates 41 (5): 3089–3108. doi:10.1007/s11164-013-1417-4.
- ↑ "Ponceau S solution". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/sial/p3504?lang=en. Retrieved 2016-11-24. "The following are two common stain formulations…"
 |
