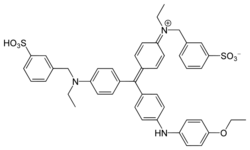
Chemistry:Coomassie brilliant blue
| |
| |
| Names | |
|---|---|
| Other names
C.I. 42660, C.I. Acid Blue 83
Brilliant indocyanine 6B, Brillantindocyanin 6B Brilliant Cyanine 6B, Serva Blue R | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C45H44N3NaO7S2 (Sodium salt) | |
| Molar mass | 825.97 g/mol |
| Insoluble in cold, slightly soluble in hot (bright red blue) | |
| Solubility in ethanol | Slightly soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
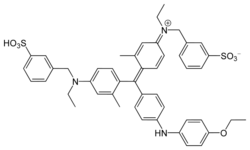
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
C.I. 42655, C.I. Acid Blue 90
Brilliant indocyanine G, Brillantindocyanin G Xylene Brilliant Cyanine G, Serva Blue G | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| KEGG | |||
PubChem CID
|
|||
| |||
| Properties | |||
| C47H50N3NaO7S2 (Sodium salt) | |||
| Molar mass | 856.03 g/mol | ||
| Slightly soluble in cold, soluble in hot (bright blue) | |||
| Solubility in ethanol | Soluble | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.
Name and discovery
The name Coomassie was adopted at the end of the 19th century as a trade name by the Blackley-based dye manufacturer Levinstein Ltd, in marketing a range of acid wool dyes.[1] In 1896 during the Fourth Anglo–Ashanti War, British forces had occupied the town of Coomassie (modern-day Kumasi in Ghana). In 1918 Levinstein Ltd became part of British Dyestuffs, which in 1926 became part of Imperial Chemical Industries.[2] Although ICI still owns the Coomassie trademark, the company no longer manufactures the dyes.
The blue disulfonated triphenylmethane dyes were first produced in 1913 by Max Weiler, who was based in Elberfeld, Germany.[3] Various patents were subsequently taken out on the organic synthesis.[4][5][6]
Papers published in biochemistry journals frequently refer to these dyes simply as "Coomassie" without specifying which dye was used. In fact, the Colour Index lists over 40 dyes with "Coomassie" in their name. There are also other Coomassie "blue" dyes. For example, the Merck Index (10th edition) lists Coomassie Blue RL (Acid Blue 92, C.I. 13390), which has a completely different structure.
Dye colour
The suffix "R" in the name of Coomassie brilliant blue R-250 is an abbreviation for "red" as the blue colour of the dye has a slight reddish tint. For the "G" variant the blue colour has a more greenish tint. The "250" originally denoted the purity of the dye.
The colour of the two dyes depends on the acidity of the solution. The "G" form of the dye has been studied in detail.[7] At a pH of less than 0 the dye has a red colour with an absorption maximum at a wavelength of 465 nm. At a pH of around 1 the dye is green with an absorption maximum at 620 nm while above pH 2 the dye is bright blue with a maximum at 595 nm. At pH 7 the dye has an extinction coefficient of 43,000 M−1 cm−1.[7]
The different colours are a result of the different charged states of the dye molecule. In the red form, all three nitrogen atoms carry a positive charge. The two sulfonic acid groups have extremely low pKa and will normally be negatively charged, thus at a pH of around zero the dye will be a cation with an overall charge of +1. The green colour corresponds to a form of the dye with no net overall charge. In neutral media (pH 7), only the nitrogen atom of the diphenylamine moiety carries a positive charge and the blue dye molecule is an anion with an overall charge of −1. The pKa values for the losses of the two protons are 1.15 and 1.82, respectively. The final proton is lost under alkaline conditions and the dye becomes pink (pKa 12.4).[7]
The dye interacts electrostatically but noncovalently with the amino and carboxyl groups of proteins. The dye molecules bind to proteins, including those in wool (keratin), to form a protein–dye complex. The formation of the complex stabilises the negatively charged anionic form of the dye, producing the blue colour, even under acid conditions when most of the molecules in solution are in the cationic form.[7] This is the basis of the Bradford assay, which quantifies protein by Coomassie brilliant blue dye binding. The binding of the dye to a protein causes a shift in the absorbance maximum of the dye from 465 to 595 nm. The increase of absorption at 595 nm is monitored to determine protein concentration.[8]
The dye also forms a complex with the anionic detergent sodium dodecylsulfate (SDS).[9] The formation of this complex stabilizes the neutral, green form of the dye. This effect can interfere with the estimation of protein concentration using the Bradford assay. It is also likely that the anionic detergent competes with the dye for binding to the protein.
Applications in biochemistry
Coomassie brilliant blue R-250 was first used to visualise proteins in 1963 by Fazekas de St. Groth and colleagues. Protein samples were separated electrophoretically on a cellulose acetate sheet. The sheet was then soaked in sulfosalicylic acid to fix the protein bands and transferred to a solution of the dye.[10]
Two years later in 1965 Meyer and Lambert used Coomassie brilliant blue R-250 to stain protein samples after electrophoretic separation in a polyacrylamide gel. They soaked the gel in a dye solution containing methanol, acetic acid and water. As the dye stained the polyacrylamide gel as well as the protein, in order to visualise the protein bands they needed to destain the gel, which they did electrophoretically.[11] Subsequent publications reported that polyacrylamide gels could be successfully destained using an acetic acid solution.
The first report of the use of the G form of the dye to visualise protein bands in polyacrylamide gels came in 1967, where the dye was dissolved in an acetic acid solution containing methanol.[12] It was subsequently discovered that the protein bands could be stained without staining the polyacrylamide by using a colloid of the G form of the dye in a trichloroacetic acid solution containing no methanol. With this procedure it was no longer necessary to destain the gel.[13] Modern formulations typically use a colloid of the G form of dye in a solution containing phosphoric acid, ethanol (or methanol) and ammonium sulfate (or aluminium sulfate).[14][15][16][17]
The Bradford assay uses the spectral properties of Coomassie brilliant blue G-250 to estimate the amount of protein in a solution.[18] A protein sample is added to a solution of the dye in phosphoric acid and ethanol. Under the acid conditions the dye is normally a brownish colour but on binding to the protein the blue form of the dye is produced. The optical absorbance of the solution is measured at a wavelength of 595 nm. The dye is noted for its high level of sensitivity: 5 μg of protein[clarification needed] can be detected. However, among the disadvantages of the method is its variability of color development with different proteins: the absorbance change per unit mass of proteins varies with the type of the protein.[19]
On binding to a protein, the negatively charged Coomassie brilliant blue G-250 dye molecule will give an overall negative charge to the protein. This property can be used to separate proteins or protein complexes using polyacrylamide gel electrophoresis under non-denaturing conditions in a technique called blue native PAGE.[20][21] The mobility of the complex in the polyacrylamide gel will depend on both the size of the protein complex (i.e., the molecular weight) and the amount of dye bound to the protein.
Coomassie blue staining can also be used as a loading control staining method in western blot analysis.[22] It is applied as an anionic pre-antibody stain.
Medical uses
In 2009, brilliant blue G was used in scientific experiments to treat spinal injuries in laboratory rats.[23] It acts by reducing the body's natural swelling response, which can cause neurons in the area to die of metabolic stress. Testing on the rats proved effective. In comparison to the rats that had not received the dye, the rats that were treated with the dye performed better on motion tests.[24] It is unknown whether this treatment can be used effectively in humans. The animal experiments administered the dye within 15 minutes of injury, but to be effective in a real-life setting, where it may take time for a patient to reach the emergency room, the treatment needs to be effective even when administered up to two hours after injury. The only reported side effect was that the rats temporarily turned blue.[23][25][26]
Under the trade names ILM Blue and Brilliant Peel, brilliant blue G is used as a stain to assist surgeons in retinal surgery.[27] In December 2019, brilliant blue G (under the trade name TissueBlue, DORC International, Netherlands) was approved for use in humans in the United States.[28][29][30]
Tissueblue was approved for medical use in Canada in January 2021.[31]
Application in forensic science
The ability of the Coomassie dye to target amino acids with aromatic groups (phenylalanine, tyrosine, tryptophan) and basic side chains (lysine, arginine and histidine) allows the Bradford assay to be used for fingerprint analysis. The assay was successfully used to identify the biological sex of the fingerprint. Female samples were shown to have a higher absorbance than male samples when tested at similar wavelengths. This provides a simpler method for fingerprint analysis by reducing the number of amino acids needing to be analyzed from 23 to 6 and requires little to no assay preparation, in contrast to the ninhydrin chemical assay, which requires assay preparation such as heating and enzyme cascade.[32]
References
- ↑ Fox, M. R. (1987). Dye-makers of Great Britain 1856-1976 : A History of Chemists, Companies, Products and Changes. Manchester: Imperial Chemical Industries. p. 38.
- ↑ Fox, M. R. (1987). Dye-makers of Great Britain 1856-1976 : A History of Chemists, Companies, Products and Changes. Manchester: Imperial Chemical Industries. p. 259.
- ↑ Colour Index. 4 (3rd ed.). Bradford: Society of Dyers and Colourists. 1971. pp. 4397–4398. http://www.colour-index.org/help/3121_Triarylmethane.pdf. Retrieved 2010-07-20.
- ↑ "Procédé de production de colorants de la série du triarylméthane" FR patent 474260, issued 1915-02-16, assigned to Bayer
- ↑ Weiler, Max, "Blue Triphenylmethane Dye", US patent 1218232, issued 1917-03-06
- ↑ "Manufacture of Triarylmethane-dyestuffs" GB patent 275609, issued 1927-11-03, assigned to IG Farbenindustrie
- ↑ 7.0 7.1 7.2 7.3 Chial, H. J.; Thompson, H. B.; Splittgerber, A. G. (1993). "A spectral study of the charge forms of Coomassie Blue G". Analytical Biochemistry 209 (2): 258–266. doi:10.1006/abio.1993.1117. PMID 7682385.
- ↑ Bradford, Marion M. (1976). "A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding". Analytical Biochemistry 72 (1–2): 248–254. doi:10.1016/0003-2697(76)90527-3. PMID 942051. http://hoffman.cm.utexas.edu/courses/bradford_assay.pdf.
- ↑ Compton, S. J.; Jones, C. G. (1985). "Mechanism of dye response and interference in the Bradford protein assay". Analytical Biochemistry 151 (2): 369–374. doi:10.1016/0003-2697(85)90190-3. PMID 4096375.
- ↑ Fazekas de St. Groth, S.; Webster, R. G.; Datyner, A. (1963). "Two new staining procedures for quantitative estimation of proteins on electrophoretic strips". Biochimica et Biophysica Acta 71: 377–391. doi:10.1016/0006-3002(63)91092-8. PMID 18421828.
- ↑ Meyer, T. S.; Lambert, B. L. (1965). "Use of Coomassie brilliant blue R250 for the electrophoresis of microgram quantities of parotid saliva proteins on acrylamide-gel strips". Biochimica et Biophysica Acta (BBA) - General Subjects 107 (1): 144–145. doi:10.1016/0304-4165(65)90403-4. PMID 4159310.
- ↑ Altschul, A. M.; Evans, W. J. (1967). Zone electrophoresis with polyacrylamide gel. Methods in Enzymology. 11. pp. 179–186. doi:10.1016/S0076-6879(67)11019-7. ISBN 9780121818609.. Page 184 personal communication from W. J. Saphonov.
- ↑ Diezel, W.; Kopperschläger, G.; Hofmann, E. (1972). "An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue". Analytical Biochemistry 48 (2): 617–620. doi:10.1016/0003-2697(72)90117-0. PMID 4115985.
- ↑ Neuhoff, V.; Stamm, R.; Eibl, H. (1985). "Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: a systematic analysis". Electrophoresis 6 (9): 427–448. doi:10.1002/elps.1150060905.
- ↑ Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G. M.; Carnemolla, B.; Orecchia, P.; Zardi, L. et al. (2004). "Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis". Electrophoresis 25 (9): 1327–1333. doi:10.1002/elps.200305844. PMID 15174055.
- ↑ Steinberg, T. H. (2009). Protein gel staining methods: an introduction and overview. Methods in Enzymology. 463. pp. 541–563. doi:10.1016/S0076-6879(09)63031-7. ISBN 9780123745361.
- ↑ Pink, M.; Verma, N.; Rettenmeier, A. W.; Schmitz-Spanke, S. (2010). "CBB staining protocol with higher sensitivity and mass spectrometric compatibility". Electrophoresis 31 (4): 593–598. doi:10.1002/elps.200900481. PMID 20162584.
- ↑ Bradford, M. M. (1976). "Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding". Analytical Biochemistry 72 (1–2): 248–254. doi:10.1016/0003-2697(76)90527-3. PMID 942051.
- ↑ Congdon, Robert W.; Muth, Gregory W.; Splittgerber, Allan G. (September 1993). "The binding interaction of Coomassie blue with proteins.". Analytical Biochemistry 213 (2): 407–413. doi:10.1006/abio.1993.1439. PMID 7694523.
- ↑ Schägger, H.; Jagow, G. (1991). "Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form". Analytical Biochemistry 199 (2): 223–231. doi:10.1016/0003-2697(91)90094-A. PMID 1812789.
- ↑ Wittig, I.; Braun, H. P.; Schägger, H. (2006). "Blue native PAGE". Nature Protocols 1 (1): 418–428. doi:10.1038/nprot.2006.62. PMID 17406264.
- ↑ Welinder, Charlotte; Ekblad, Lars (2011). "Coomassie Staining as Loading Control in Western Blot Analysis". Journal of Proteome Research 10 (3): 1416–1419. doi:10.1021/pr1011476. PMID 21186791.
- ↑ 23.0 23.1 Peng, W.; Cotrina, M. L.; Han, X.; Yu, H.; Bekar, L.; Blum, L.; Takano, T.; Tian, G. F. et al. (2009). "Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury". Proceedings of the National Academy of Sciences of the United States of America 106 (30): 12489–12493. doi:10.1073/pnas.0902531106. PMID 19666625.
- ↑ Ehrenberg, Rachel (23 September 2013). "Brilliant blue for the spine" (in en). Society for Science & the Public. https://www.sciencenews.org/article/brilliant-blue-spine.
- ↑ "Blue M&Ms 'mend spinal injuries'". Telegraph. 2009-07-28. https://www.telegraph.co.uk/news/science/science-news/5921266/Blue-MandMs-mend-spinal-injuries.html.
- ↑ "Blue Food Dye Treats Spine Injury in Rats". Wired.com. 2009-07-27. https://www.wired.com/wiredscience/2009/07/bluerats/.
- ↑ Mennel, S.; Meyer, C. H.; Schmidt, J. C.; Kaempf, S.; Thumann, G. (2008). Trityl dyes patent blue V and brilliant blue G - clinical relevance and in vitro analysis of the function of the outer blood-retinal barrier. Developments in Ophthalmology. 42. pp. 101–114. doi:10.1159/000138988. ISBN 978-3-8055-8551-4.
- ↑ "Drug Trials Snapshots: TissueBlue". 20 December 2019. http://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-tissueblue.
- ↑ "TissueBlue- brilliant blue g injection, solution". 29 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d4b28b4-c582-4e2c-abd9-f7e203378e2d.
- ↑ "Drug Approval Package: TissueBlue". 10 January 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209569Orig1s000TOC.cfm.
- ↑ "Summary Basis of Decision (SBD) for Tissueblue". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00532&lang=en.
- ↑ Brunelle, Erica; Le, Anh Minh; Huynh, Crystal; Wingfield, Kelly; Halámková, Lenka; Agudelo, Juliana; Halámek, Jan (2017-04-04). "Coomassie Brilliant Blue G-250 Dye: An Application for Forensic Fingerprint Analysis". Analytical Chemistry 89 (7): 4314–4319. doi:10.1021/acs.analchem.7b00510. ISSN 0003-2700. PMID 28293949.
Further reading
- Gessner, T.; Mayer, U. (2002). "Triarylmethane and Diarylmethane Dyes". Ullmann's Encyclopedia of Industrial Chemistry 6th Edition. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179. ISBN 978-3527306732.
External links
- "Brilliant Blue G". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/6104-58-1.
- "Food dye 'may ease spinal injury'". 28 July 2009. https://news.bbc.co.uk/2/hi/health/8170033.stm.
 |

