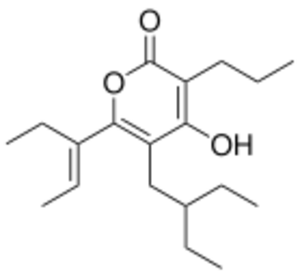
Chemistry:Prescopranone
| |
| Names | |
|---|---|
| IUPAC name
(E)-3-ethyl-5-(2-ethylbutyl)-4-hydroxy-6-(pent-2-en-3-yl)-2H-pyran-2-one
| |
| Identifiers | |
| |
| Properties | |
| C18H28O3 | |
| Molar mass | 292.419 g·mol−1 |
| Appearance | Pale yellow oil |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Prescopranone is a key intermediate in the biosynthesis of scopranones.[1] Prescopranone is the precursor to scopranone A, scopranone B, and scopranone C, which are produced by Streptomyces sp. BYK-11038.
Biosynthesis
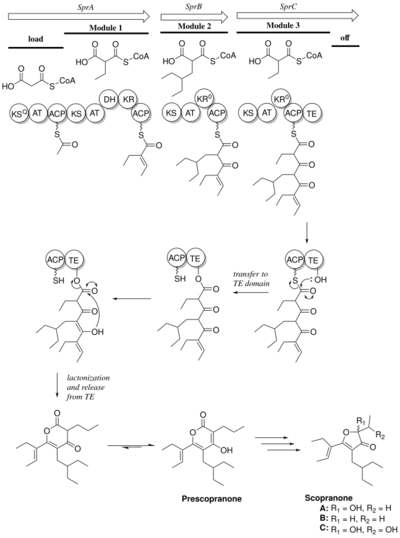
The biosynthesis of prescopranone follows the module structure of a Type I polyketide synthase(PKS) with three elongation modules and a lactonizing thioesterase domain. Genome mining of Streptomyces sp. BYK identified a scopranone biosynthetic gene cluster containing 3 genes, sprA, sprB, and sprC, that encode modular PKSs.[2]
A starter acyl carrier protein is loaded with malonyl-CoA, and decarboxylated by a ketosynthase (KSQ). The starter unit is then transferred to module 1, which elongates the polyketide chain with ethyl malonyl-CoA. The tailoring domain of this module reduces the β-carbonyl to an alkene. Module 2 elongates the polyketide with ethylbutyl-malonyl-CoA. Finally, module 3 elongates the polyketide chain with ethyl malonyl-CoA, and is released upon the lactonization of the polyketide product by a thioesterase domain. Following the cyclization and release of the polyketide, the product undergoes a keto-enol tautomerism to form prescopranone. Both modules 2 and 3 contain dysfunctional ketoreductase (KR) domains, which do not reduce the β-carbonyl due to missing NAD(P)H binding motifs and tyrosine residues in their active sites.[2] Prescopranone undergoes post-PKS transformations to form scopranones. Additionally, the deletion of a downstream gene sprT can disrupt biosynthesis of scopranones in Streptomyces avermitilis SUKA54. The products of this mutated pathway have yet to be elucidated.[3]
Research
Prescopranone and similar compounds are currently being investigated as bone morphogenetic protein (BMP) inhibitors for the treatment of fibrodysplasia ossificans progressiva (FOP).[2][3]
References
- ↑ "Scopranones with Two Atypical Scooplike Moieties Produced by Streptomyces sp. BYK-11038.". Org. Lett. 19 (21): 5980–5983. 2017. doi:10.1021/acs.orglett.7b03003. PMID 29063763.
- ↑ 2.0 2.1 2.2 "An Unusual Extender Unit Is Incorporated into the Modular Polyketide Synthase of Scopranones Biosynthesis". Biochemistry 58 (50): 5066–5073. 2019. doi:10.1021/acs.biochem.9b00908. PMID 31756295.
- ↑ 3.0 3.1 "Discovery of prescopranone, a key intermediate in scopranone biosynthesis.". The Journal of Antibiotics 75 (6): 305–311. 2022. doi:10.1038/s41429-022-00521-x. PMID 35444295.
 |
