Chemistry:Proline organocatalysis
Proline organocatalysis is the use of proline as an organocatalyst in organic chemistry. This theme is often considered the starting point for the area of organocatalysis, even though early discoveries went unappreciated.[1] Modifications, such as MacMillan’s catalyst and Jorgensen's catalysts, proceed with excellent stereocontrol.[2]:5574[3] Proline catalysis was initially reported by groups at Schering AG and Hoffmann-La Roche.[1][4][5][6] Proline's chiral structure enables enantioselective synthesis, favoring a particular enantiomer or diastereomer.[2]:5574[1][7][8][9]:47
Reactions
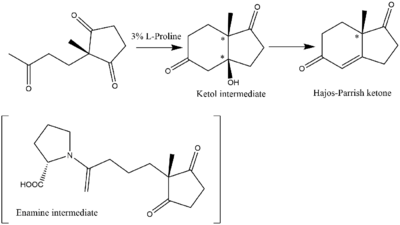
The Hajos–Parrish–Eder–Sauer–Wiechert reaction, reported in 1971 by several research teams, is an early example of an enantioselective catalytic reaction in organic chemistry.[10] Its scope has been modified and expanded through the development of related reactions including the Michael addition, asymmetric aldol reaction, and the Mannich reaction. This reaction has likewise been used to perform asymmetric Robinson annulations. The general scheme of this reaction follows:
This example illustrates a 6-enolendo aldolization. In the , proline catalyses an asymmetric aldol reaction. The zwitterionic character and the H-bonding of proline in the transition state determine the reaction outcome.[11][12][13][14] An enamine is formed during the reaction and only one proline molecule is involved in forming the transition state.[15]
Asymmetric synthesis of the Wieland-Miescher ketone is also based on proline.[16] Additional reactions include aldol reactions,[17][18][19][20] Mannich reaction,[21][22][23] Michael reaction,[24][25] amination,[22] α-oxyamination,[26][27] and α-halogenation.[28][29]
Modifications on the basic proline structure improved the enantioselectivity and regioselectivity of the catalysis.[28][29] These proline-derived auxiliaries and catalysts,[30] including the Enders hydrazone reaction and Corey–Itsuno reduction, have been reviewed,[31][32] as have MacMillan’s iminium catalysts,[33] Miller catalysts,[33] and CBS-oxazaborolidines.[34]
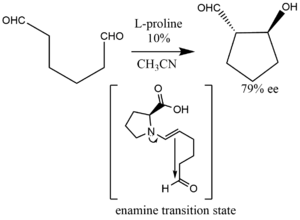
Illustrating an enolexo intramolecular aldolization, dicarbonyl (dials,diketones) can be converted to anti-aldol products with a 10% L-proline catalyst loading.[35][36]
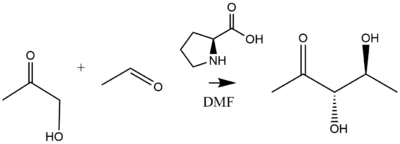
A prominent example of proline catalysis is the addition of acetone or hydroxyacetone to a diverse set of aldehydes catalyzed by 20-30% proline catalyst loading with high (>99%) enantioselectivity yielding diol products.[37] As refined by List and Notz, the aforementioned reaction produces diol products as follows:[38]
Mechanistic considerations
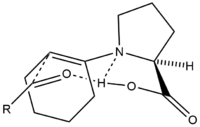
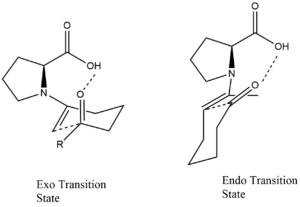
Proline-catalyzed aldol additions proceed via a six-membered enamine transition state according to the Zimmerman-Traxler model. Addition of 20-30 mol% proline to acetone or hydroxyacetone catalyzes their addition to a diverse set of aldehydes with high (>99%) enantioselectivity yielding diol products.[39] [40] [41] Proline and proline derivatives have been implemented as organocatalysts to promote asymmetric condensation reactions. An example of such a reaction proceeding through a six membered transition state is modelled as follows.
Intramolecular aldolization reactions that are catalyzed by proline likewise go through six-membered transition states. These transition states can enable the formation of either the enolexo or the enolendo product.[42]
References
- ↑ 1.0 1.1 1.2 Gaunt, M. J.; Johansson, C. C. C.; McNally, A.; Vo, N. T. (2007). "Enantioselective organocatalysis". Drug Discovery Today 12 (1–2): 8–27. doi:10.1016/j.drudis.2006.11.004. PMID 17198969.
- ↑ 2.0 2.1 List, B. (2002). "Proline-catalyzed asymmetric reactions". Tetrahedron 58 (28): 5573–5590. doi:10.1016/S0040-4020(02)00516-1.
- ↑ Wang, Z. (2009). In "Comprehensive Organic Name Reactions and Reagents", page 1306, John Wiley & Sons. ISBN:0-471-70450-4, ISBN:978-0-471-70450-8
- ↑ Hajos, Z. G. and Parrish, D. R. (1971) German Patent DE 2102623
- ↑ Eder, U.; Sauer, G.; Wiechert, R. (1971). "New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures". Angewandte Chemie International Edition in English 10 (7): 496–497. doi:10.1002/anie.197104961.
- ↑ Hajos, Z. G.; Parrish, D. R. (1974). "Synthesis and conversion of 2-methyl-2-(3-oxobutyl)-1,3-cyclopentanedione to the isomeric racemic ketols of the \3.2.1]bicyclooctane and of the perhydroindane series". The Journal of Organic Chemistry 39 (12): 1612. doi:10.1021/jo00925a002.
- ↑ Dalko, P.; Moisan, L. (2001). "Enantioselective Organocatalysis". Angewandte Chemie International Edition 40 (20): 3726–3748. doi:10.1002/1521-3773(20011015)40:20<3726::AID-ANIE3726>3.0.CO;2-D. PMID 11668532.
- ↑ Berkessel, A., Groeger, H. (2005). "Asymmetric Organocatalysis". Wiley-VCH ISBN:3-527-30517-3
- ↑ Dalko, P.I. (editor) (2007). "Enantioselective Organocatalysis: Reactions and Experimental Procedures". John Wiley & Sons. ISBN:978-3-527-31522-2
- ↑ Wang, Zerong (2009). Comprehensive organic name reactions and reagents. Hoboken, N.J.: John Wiley. ISBN 978-0-470-63885-9.
- ↑ Hajos, Z. G.; Parrish, D. R. (1974). "Asymmetric synthesis of bicyclic intermediates of natural product chemistry". The Journal of Organic Chemistry 39 (12): 1615–1621. doi:10.1021/jo00925a003.
- ↑ Clemente, F. R.; Houk, K. N. (2004). "Computational Evidence for the Enamine Mechanism of Intramolecular Aldol Reactions Catalyzed by Proline". Angewandte Chemie 116 (43): 5890. doi:10.1002/ange.200460916. Bibcode: 2004AngCh.116.5890C.
- ↑ List, B.; Hoang, L.; Martin, H. J. (2004). "Asymmetric Catalysis Special Feature Part II: New mechanistic studies on the proline-catalyzed aldol reaction". Proceedings of the National Academy of Sciences 101 (16): 5839–5842. doi:10.1073/pnas.0307979101. PMID 15073330. Bibcode: 2004PNAS..101.5839L.
- ↑ Rankin, K. N.; Gauld, J. W.; Boyd, R. J. (2002). "Density Functional Study of the Proline-Catalyzed Direct Aldol Reaction". The Journal of Physical Chemistry A 106 (20): 5155. doi:10.1021/jp020079p. Bibcode: 2002JPCA..106.5155R.
- ↑ Hoang, L.; Bahmanyar, S.; Houk, K. N.; List, B. (2003). "Kinetic and Stereochemical Evidence for the Involvement of Only One Proline Molecule in the Transition States of Proline-Catalyzed Intra- and Intermolecular Aldol Reactions". Journal of the American Chemical Society 125 (1): 16–17. doi:10.1021/ja028634o. PMID 12515489.
- ↑ Woodward, R. B.; Logusch, E.; Nambiar, K. P.; Sakan, K.; Ward, D. E.; Au-Yeung, B. W.; Balaram, P.; Browne, L. J. et al. (1981). "Asymmetric total synthesis of erythromcin. 1. Synthesis of an erythronolide a secoacid derivative via asymmetric induction". Journal of the American Chemical Society 103 (11): 3210. doi:10.1021/ja00401a049.
- ↑ Northrup, A. B.; MacMillan, D. W. C. (2002). "The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes". Journal of the American Chemical Society 124 (24): 6798–6799. doi:10.1021/ja0262378. PMID 12059180. https://authors.library.caltech.edu/76965/2/ja0262378_s.pdf.
- ↑ Notz, W.; List, B. (2000). "Catalytic Asymmetric Synthesis ofanti-1,2-Diols". Journal of the American Chemical Society 122 (30): 7386. doi:10.1021/ja001460v.
- ↑ List, B.; Pojarliev, P.; Castello, C. (2001). "Proline-Catalyzed Asymmetric Aldol Reactions between Ketones and α-Unsubstituted Aldehydes". Organic Letters 3 (4): 573–575. doi:10.1021/ol006976y. PMID 11178828.
- ↑ List, B.; Lerner, R. A.; Barbas 3rd, C. F. (2000). "Proline-Catalyzed Direct Asymmetric Aldol Reactions". Journal of the American Chemical Society 122 (10): 2395. doi:10.1021/ja994280y.
- ↑ Córdova, A.; Watanabe, S.; Tanaka, F.; Notz, W.; Barbas 3rd, C.F. (2002). "A highly enantioselective route to either enantiomer of both alpha- and beta-amino acid derivatives". Journal of the American Chemical Society 124 (9): 1866–1867. doi:10.1021/ja017833p. PMID 11866595.
- ↑ 22.0 22.1 List, B.; Pojarliev, P.; Biller, W. T.; Martin, H. J. (2002). "The Proline-Catalyzed Direct Asymmetric Three-Component Mannich Reaction: Scope, Optimization, and Application to the Highly Enantioselective Synthesis of 1,2-Amino Alcohols". Journal of the American Chemical Society 124 (5): 827–833. doi:10.1021/ja0174231. PMID 11817958.
- ↑ Marques, M. M. B. (2006). "Catalytic Enantioselective Cross-Mannich Reaction of Aldehydes". Angewandte Chemie International Edition 45 (3): 348–352. doi:10.1002/anie.200502630. PMID 16342308.
- ↑ List, B.; Pojarliev, P.; Martin, H. J. (2001). "Efficient Proline-Catalyzed Michael Additions of Unmodified Ketones to Nitro Olefins". Organic Letters 3 (16): 2423–2425. doi:10.1021/ol015799d. PMID 11483025.
- ↑ List, B.; Castello, C. (2001). "A Novel Proline-Catalyzed Three-Component Reaction of Ketones, Aldehydes, and Meldrum's Acid". Synlett 2001 (11): 1687. doi:10.1055/s-2001-18095.
- ↑ Zhong, G. (2003). "A Facile and Rapid Route to Highly Enantiopure 1,2-Diols by Novel Catalytic Asymmetricα-Aminoxylation of Aldehydes". Angewandte Chemie International Edition 42 (35): 4247–4250. doi:10.1002/anie.200352097. PMID 14502748.
- ↑ Brown, S. P.; Brochu, M. P.; Sinz, C. J.; MacMillan, D. W. C. (2003). "The Direct and Enantioselective Organocatalytic α-Oxidation of Aldehydes". Journal of the American Chemical Society 125 (36): 10808–10809. doi:10.1021/ja037096s. PMID 12952459. https://authors.library.caltech.edu/76698/2/ja037096ssi20030802_025802.pdf.
- ↑ 28.0 28.1 Brochu, M. P.; Brown, S. P.; MacMillan, D. W. C. (2004). "Direct and Enantioselective Organocatalytic α-Chlorination of Aldehydes". Journal of the American Chemical Society 126 (13): 4108–4109. doi:10.1021/ja049562z. PMID 15053591. https://authors.library.caltech.edu/76770/2/ja049562zsi20040223_021457.pdf.
- ↑ 29.0 29.1 Franzén, J.; Marigo, M.; Fielenbach, D.; Wabnitz, T. C.; Kjaersgaard, K. A.; Jørgensen, K. A. (2005). "A General Organocatalyst for Direct α-Functionalization of Aldehydes: Stereoselective C−C, C−N, C−F, C−Br, and C−S Bond-Forming Reactions. Scope and Mechanistic Insights". Journal of the American Chemical Society 127 (51): 18296–18304. doi:10.1021/ja056120u. PMID 16366584.
- ↑ Cobb, A. J. A.; Shaw, D. M.; Longbottom, D. A.; Gold, J. B.; Ley, S. V. (2005). "Organocatalysis with proline derivatives: Improved catalysts for the asymmetric Mannich, nitro-Michael and aldol reactions". Organic & Biomolecular Chemistry 3 (1): 84–96. doi:10.1039/b414742a. PMID 15602602.
- ↑ Job, A.; Janeck, C. F.; Bettray, W.; Peters, R.; Enders, D. (2002). "The SAMP-/RAMP-hydrazone methodology in asymmetric synthesis". Tetrahedron 58 (12): 2253. doi:10.1016/S0040-4020(02)00080-7.
- ↑ Corey, E. J.; Helal, C. J. (1998). "Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method". Angewandte Chemie International Edition 37 (15): 1986–2012. doi:10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. PMID 29711061.
- ↑ 33.0 33.1 Jarvo, E. R.; Miller, S. J. (2002). "Amino acids and peptides as asymmetric organocatalysts". Tetrahedron 58 (13): 2481. doi:10.1016/S0040-4020(02)00122-9.
- ↑ Stemmler, R. (2007). "CBS Oxazaborolidines - Versatile Catalysts for Asymmetric Synthesis". Synlett 2007 (6): 0997–0998. doi:10.1055/s-2007-973876.
- ↑ Pidathala, Chandarakala; Hoang, Linh; Vignola, Nicola; List, Benjamin (2003). "Direct Catalytic Asymmetric Enolexo Aldolization". Angewandte Chemie International Edition 42 (24): 2785–2788. doi:10.1002/anie.200351266. PMID 12820268.
- ↑ Mukherjee, Santanu; Yang, Jung; Hoffmann, Sebastian; List, Benjamin (2007). "Asymmetric Enamine Catalysis". Chem. Rev. 107 (12): 5471–5569. doi:10.1021/cr0684016. PMID 18072803.
- ↑ Garcia, Jesus; Oiarbide, Mikel; Palomo, Claudio (15 July 2005). "Current Progress in the asymmetric aldol addition reaction". Chem. Soc. Rev. 33 (2): 65–75. doi:10.1039/b202901d. PMID 14767502.
- ↑ Notz, W; List, B. (2000). "Proline-Catalyzed Direct Asymmetric Aldol Reactions". Journal of the American Chemical Society 122 (10): 2395. doi:10.1021/ja994280y.
- ↑ Garcia, Jesus; Oiarbide, Mikel; Palomo, Claudio (15 July 2005). "Current Progress in the asymmetric aldol addition reaction". Chem. Soc. Rev. 33 (2): 65–75. doi:10.1039/b202901d. PMID 14767502.
- ↑ Notz, W; List, B. (2000). "Proline-Catalyzed Direct Asymmetric Aldol Reactions". Journal of the American Chemical Society 122 (10): 2395. doi:10.1021/ja994280y.
- ↑ Sakthivel, K.; Notz, W; Bui, T; Barbas, C (2000). "Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: A Bioorganic Approach to Catalytic Asymmetric Carbon−Carbon Bond-Forming Reactions". Journal of the American Chemical Society 122 (22): 5260–5267. doi:10.1021/ja010037z. PMID 11457388.
- ↑ Garcia, Jesus; Oiarbide, Mikel; Palomo, Claudio (15 July 2005). "Current Progress in the asymmetric aldol addition reaction". Chem. Soc. Rev. 33 (2): 65–75. doi:10.1039/b202901d. PMID 14767502.
 |