Chemistry:Mannich reaction
| Mannich reaction | |
|---|---|
| Named after | Carl Mannich |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | mannich-reaction |
| RSC ontology ID | RXNO:0000032 |
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl (C=O) functional group by formaldehyde (H–CHO) and a primary or secondary amine (–NH
2) or ammonia (NH
3).[1] The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes.
The reaction is named after Carl Mannich.[2][3]
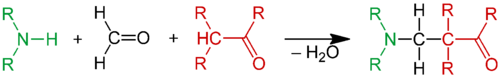
The Mannich reaction starts with the nucleophilic addition of an amine to a carbonyl group followed by dehydration to the Schiff base. The Schiff base is an electrophile which reacts in a second step in an electrophilic addition with an enol formed from a carbonyl compound containing an acidic alpha-proton. The Mannich reaction is a condensation reaction.[4]:140
In the Mannich reaction, primary or secondary amines or ammonia react with formaldehyde to form a Schiff base. Tertiary amines lack an N–H proton and so do not react. The Schiff base can react with α-CH-acidic compounds (nucleophiles) that include carbonyl compounds, nitriles, acetylenes, aliphatic nitro compounds, α-alkyl-pyridines or imines. It is also possible to use activated phenyl groups and electron-rich heterocycles such as furan, pyrrole, and thiophene. Indole is a particularly active substrate; the reaction provides gramine derivatives.
The Mannich reaction can be considered to involve a mixed-aldol reaction, dehydration of the alcohol, and conjugate addition of an amine (Michael reaction) all happening in "one-pot". Double Mannich reactions can also occur.
Reaction mechanism
The mechanism of the Mannich reaction starts with the formation of an iminium ion from the amine and formaldehyde.[4]:140
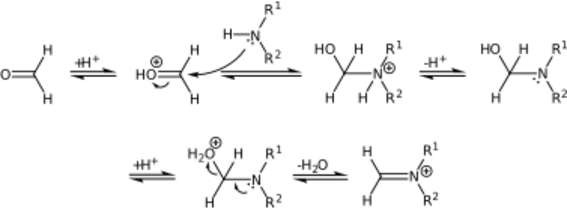
The compound with the carbonyl functional group (in this case a ketone) will tautomerize to the enol form, after which it attacks the iminium ion.
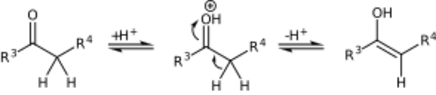
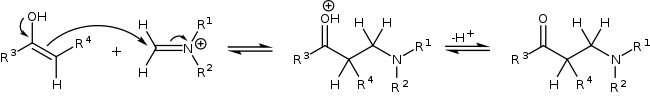
On methyl ketones, the enolization and the Mannich addition can occur twice, followed by an β-elimination to yield β-amino enone derivatives.[5][6]
Asymmetric Mannich reactions
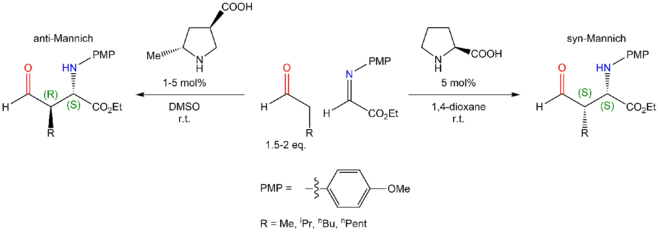
(S)-proline catalyzes a chiral Mannich reaction. It diastereoselects the syn adduct, with greater effect for larger aldehyde substituents; and enantioselects the (S, S) adduct.[7] A substituted proline can instead catalyze the (R, S) anti adduct.[8]
Applications
The Mannich reaction is used in many areas of organic chemistry, Examples include:
- alkyl amines
- peptides, nucleotides, antibiotics, and alkaloids (e.g. tropinone[4]:142)
- agrochemicals, such as plant growth regulators[9]
- polymers
- catalysts
- Formaldehyde tissue crosslinking
- Pharmaceutical drugs (e.g. rolitetracycline (the Mannich product of tetracycline and pyrrolidine), fluoxetine (antidepressant), tramadol and tolmetin (anti-inflammatory drug).
- soap and detergents, especially with application to automotive fuel[10]
- Polyetheramines from substituted branched chain alkyl ethers.[11][12]
- α,β-unsaturated ketones by the thermal degradation of Mannich reaction products (e.g. methyl vinyl ketone from 1-diethylamino-butan-3-one)[13][14]
See also
- Betti reaction
- Kabachnik–Fields reaction
- Pictet–Spengler reaction
- Stork enamine alkylation
- Nitro-Mannich reaction
References
- ↑ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. pp. 1292–1295. ISBN 978-0-471-72091-1.
- ↑ Carl Mannich; Krösche, W. (1912). "Ueber ein Kondensationsprodukt aus Formaldehyd, Ammoniak und Antipyrin" (in German). Archiv der Pharmazie 250 (1): 647–667. doi:10.1002/ardp.19122500151. https://zenodo.org/record/1424583.
- ↑ Blicke, F. F. (2011). "The Mannich Reaction". Organic Reactions 1 (10): 303–341. doi:10.1002/0471264180.or001.10. ISBN 978-0471264187.
- ↑ 4.0 4.1 4.2 Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry: Part B: Reactions and Synthesis (5th ed.). New York: Springer. pp. 140–142. ISBN 978-0387683546.
- ↑ Cromwell, Norman H.; Soriano, David S.; Doomes, Earl (November 1980). "Mobile keto allyl systems. 18. Synthesis and chemistry of N-substituted and N,N-disubstituted 2-benzoyl-1-amino-3-propenes". The Journal of Organic Chemistry 45 (24): 4983–4985. doi:10.1021/jo01312a034.
- ↑ Girreser, Ulrich; Heber, Dieter; Schütt, Martin (May 1998). "A Facile One-Pot Synthesis of 1-Aryl-2-(dimethylaminomethyl)prop-2-en-1-ones from Aryl Methyl Ketones". Synthesis 1998 (5): 715–717. doi:10.1055/s-1998-2056.
- ↑ Córdova, A.; Watanabe, S.-I.; Tanaka, F.; Notz, W.; Barbas, C. F. (2002). "A highly enantioselective route to either enantiomer of both α- and β-amino acid derivatives". Journal of the American Chemical Society 124 (9): 1866–1867. doi:10.1021/ja017833p. PMID 11866595.
- ↑ Mitsumori, S.; Zhang, H.; Cheong, P. H.-Y.; Houk, K.; Tanaka, F.; Barbas, C. F. (2006). "Direct asymmetric anti-Mannich-type reactions catalyzed by a designed amino acid". Journal of the American Chemical Society 128 (4): 1040–1041. doi:10.1021/ja056984f. PMID 16433496.
- ↑ da Rosa, F. A. F.; Rebelo, R. A.; Nascimento, M. G. (2003). "Synthesis of new indolecarboxylic acids related to the plant hormone indoleacetic acid". Journal of the Brazilian Chemical Society 14 (1): 11–15. doi:10.1590/S0103-50532003000100003. http://www.scielo.br/pdf/jbchs/v14n1/a03v14n1.pdf.
- ↑ Aradi, Allen A.; Colucci, William J.; Scull, Herbert M.; Openshaw, Martin J. (June 19–22, 2000). "A Study of Fuel Additives for Direct Injection Gasoline (DIG) Injector Deposit Control". CEC/SAE Spring Fuels & Lubricants Meeting & Exposition. Warrendale, PA: CEC and SAE International. doi:10.4271/2000-01-2020. 2000-01-2020. http://papers.sae.org/2000-01-2020/. Retrieved 20 August 2023.
- ↑ Wang, Wenying; Wang, Wei; Zhu, Zhongpeng; Hu, Xiaoming; Qiao, Fulin; Yang, Jing; Liu, Dan; Chen, Pu et al. (2023-04-15). "Quantitation of polyetheramines as the active components of detergent additives in gasoline by the ninhydrin reaction". Fuel 338: 127275. doi:10.1016/j.fuel.2022.127275. ISSN 0016-2361. https://www.sciencedirect.com/science/article/pii/S0016236122040996.
- ↑ Kuo, Chung-Hao; Smocha, Ruth; Loeper, Paul; Mukkada, Nicholas; Simpson Green, Felicia (2022-08-30). "Aftermarket Fuel Additives and their Effects on GDI Injector Performance and Particulate Emissions" (in en). SAE Technical Paper Series (400 Commonwealth Drive, Warrendale, PA, United States: SAE International). doi:10.4271/2022-01-1074. https://doi.org/10.4271/2022-01-1074.
- ↑ Siegel, H.; Eggersdorfer, M.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.
- ↑ Wilds, A. L.; Nowak, R. M.; McCaleb, K. E. (1957). "1-Diethylamino-3-butanone (2-Butanone, 4-diethylamino-)". Organic Syntheses 37: 18. doi:10.15227/orgsyn.037.0018. http://www.orgsyn.org/demo.aspx?prep=CV4P0281.; Collective Volume, 4, pp. 281
External links
 |

