Chemistry:Prostanoic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
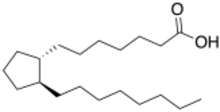
7-[(1S,2S)-2-Octylcyclopentyl]heptanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H38O2 | |
| Molar mass | 310.522 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Prostanoic acid (7-[(1S,2S)-2-octylcyclopentyl]heptanoic acid) is a saturated fatty acid which contains a cyclopentane ring. Its derivatives are prostaglandins - physiologically active lipid substances. Prostanoic acid is not found in nature, but it can be synthesized in vitro.
Synthesis
For the first time the synthesis of prostanoic acid from 1-formylcyclopentene was considered in detail in the scientific literature in 1975 by a group of French pharmacists.[1] One year later, a group of Japanese scientists, who worked in the central research laboratory of the "Sankyo Co., Ltd." company (Shinagawa, Tokyo), published another method for obtaining prostanoic acid from 2-[4-hydroxy-5-(methoxymethyl)cyclopent-2-en-1-yl] acetic acid.[2] In 1986, a group of Japanese scientists from Kyushu University in Fukuoka proposed their own scheme for obtaining prostanoic acid from limonene.[3]
See also
- Prostaglandin
- Saturated fat
- Fatty acid
- Fatty acid synthesis
- List of saturated fatty acids
- List of unsaturated fatty acids
References
- ↑ Hamon A; Lacoume B; Olivier A; Pilgrim W.R. (November 1975). "Synthesis of prostanoic acid". Tetrahedron Letters 16 (50): 4481–4482. doi:10.1016/S0040-4039(00)91098-0.
- ↑ "Synthesis of (+)-prostanoic acid (1)". Prostaglandins 12 (3): 399–401. September 1976. doi:10.1016/0090-6980(76)90020-4. PMID 968053.
- ↑ "Conversion of limonene to prostanoic acid and 8-isoprostanoic acid". Chemical and Pharmaceutical Bulletin 34 (2): 550–557. February 1986. doi:10.1248/cpb.34.550.
 |
